Action and use
Glycylcycline antibacterial.
DEFINITION
Tigecycline for Infusion is a sterile material consisting of Tigecycline with or without excipients. It is supplied in a sealed container.
The contents of the sealed container comply with the requirements for Powders for Injections or Infusions stated under
Parenteral Preparations and with the following requirements.
Content of tigecycline, C29H39N5O8
95.0 to 110.0% of the stated amount.
IDENTIFICATION
The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum produced with tigecycline EPCRS. If the spectra show differences, record a new spectrum after recrystallisation from methanol.
TESTS
Acidity
pH of a 1% w/v solution, 5.0 to 6.5. Appendix V L.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions in a solution containing 0.44% w/v of dipotassium hydrogen orthophosphate and 0.05% w/v of sodium hydrogensulfite in water, adjusted to pH 8.0 with 1M potassium hydroxide. Store solutions at 10° and protect from light. Use solutions within 12 hours of preparation.
(1) Dissolve a quantity of the contents of the sealed container to produce a solution containing 0.05% w/v of Tigecycline.
(2) Dilute 1 volume of solution (1) to 100 volumes.
(3) Dilute 1 volume of solution (2) to 10 volumes.
(4) 0.05% w/v of tigecycline for system suitability EPCRS (impurity A), 0.00024% w/v of tigecycline impurity B EPCRS and 0.00024% w/v of minocycline hydrochloride BPCRS (impurity C).
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (15 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (3 μm) (Luna C18 is suitable).
(b) Use gradient elution and the mobile phase described below.
(c) Use a flow rate of 1.0 mL per minute.
(d) Use a column temperature of 30°.
(e) Use an autosampler temperature of 10°.
(f) Use a detection wavelength of 248 nm.
(g) Inject 25 μL of each solution.
MOBILE PHASE
Mobile phase A 50 volumes of acetonitrile, 950 volumes of a solution containing 0.46% w/v of dipotassium hydrogen orthophosphate and 0.10% w/v of disodium edetate in water, previously adjusted to pH 6.4 with orthophosphoric acid.
Mobile phase B 500 volumes of acetonitrile, 500 volumes of a solution containing 0.87% w/v of dipotassium hydrogen orthophosphate and 0.19% w/v of disodium edetate in water, previously adjusted to pH 6.4 with orthophosphoric acid.
| Time (Minutes) | Mobile phase A (% v/v) | Mobile phase B (% v/v) | Comment |
| 0-2 | 85 | 15 | isocratic |
| 2-42 | 85→57 | 15→43 | linear gradient |
| 42-57 | 57→0 | 43→100 | linear gradient |
| 57-60 | 0 | 100 | isocratic |
| 60-61 | 0→85 | 100→15 | linear gradient |
| 61-68 | 85 | 15 | re-equilibration |
When the chromatograms are recorded under the prescribed conditions, the relative retentions with reference to tigecycline (retention time about 20 minutes) are: impurity 1, about 0.5; impurity B, about 0.6; impurity A, about 0.7; impurity 2, about 1.3; impurity C, about 1.6; impurity 3, about 1.7.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (4), the resolution between the peaks due to impurity B and impurity A is at least 1.5.
LIMITS
In the chromatogram obtained with solution (1):
the area of any peak corresponding to impurity A is not greater than twice the area of the principal peak in the chromatogram obtained with solution (2) (2%);
the area of any peak corresponding to impurity B is not greater than 0.7 times the area of the principal peak in the chromatogram obtained with solution (2) (0.7%);
the area of any peak corresponding to impurity C is not greater than twice the area of the principal peak in the chromatogram obtained with solution (3) (0.2%);
the area of any peak corresponding to impurity 2 is not greater than three times the area of the principal peak in the chromatogram obtained with solution (3) (0.3%);
the area of any peak corresponding to impurity 1 or impurity 3 is not greater than half the area of the principal peak in the chromatogram obtained with solution (2) (0.5% of each);
the area of any other secondary peak is not greater than twice the area of the principal peak in the chromatogram obtained with solution (3) (0.2%);
the sum of the areas of any secondary peaks is not greater than four times the area of the principal peak in the chromatogram obtained with solution (2) (4%).
Disregard any peak with an area less than the area of the principal peak in the chromatogram obtained with solution (3) (0.1%).
Water
Not more than 3.5%, Appendix IX C, Method III. Use the contents of one vial.
ASSAY
Determine the weight of the contents of 10 containers as described in the test for uniformity of weight, Appendix XII C1, Powders for Parenteral Administration.
Carry out the method for liquid chromatography, Appendix III D, using the following solutions in a solution of 0.44% w/v of dipotassium hydrogen orthophosphate and 0.05% w/v of sodium hydrogensulfite in water, adjusted to pH 8.0 with 1M potassium hydroxide. Store solutions at 10° and protected from light.
(1) Dissolve a quantity of the mixed contents of 10 containers to produce a solution containing 0.01% w/v of Tigecycline.
(2) 0.01% w/v of tigecycline EPCRS.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (15 cm × 4.6 mm) packed with end-capped octadecylsilyl silica gel for chromatography (5 μm) (Prodigy ODS2 is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1.0 mL per minute.
(d) Use a column temperature of 30°.
(e) Use an autosampler temperature of 10°.
(f) Use a detection wavelength of 248 nm.
(g) Inject 20 μL of each solution.
MOBILE PHASE
140 volumes of acetonitrile and 860 volumes of a solution of 0.44% w/v of dipotassium hydrogen orthophosphate and 0.093% w/v of disodium edetate in water, previously adjusted to pH 6.2 with orthophosphoric acid.
DETERMINATION OF CONTENT
Calculate the content of tigecycline, C29H39N5O8 in a container of average content weight from the chromatograms obtained, using the declared content of C29H39N5O8 in tigecycline EPCRS.
IMPURITIES
The impurities limited by the requirements of this monograph include those listed under Tigecycline and the following:
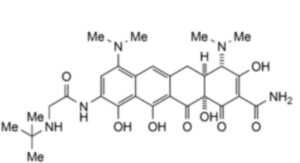
1. (4S,4aS,12aS)-9-[(tert-Butylamino)acetamido]-4,7 bis(dimethylamino)-3,10,11,12a-tetrahydroxy-1,12-dioxo-1,4,4a,5,12,12a-hexahydrotetracene-2-carboxamide
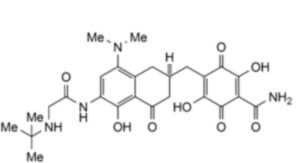
2. 4-{[(2R)-6-[(tert-Butylamino)acetamido]-8-(dimethylamino)-5-hydroxy-4-oxo-1,2,3,4-tetrahydronaphthalen-2-yl]methyl}-2,5-dihydroxy-3,6-dioxocyclohexa-1,4-diene-1-carboxamide
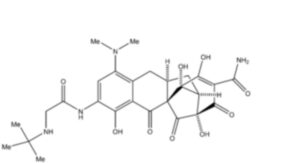
3. (1S,4aR,4bR,10aR,11aS)-7-[(tert-Butylamino)acetamido]-9-(dimethyamino)-1,4,4a,6-tetrahydroxy-2,5,12-trioxo-1,2,4a,5,10,10a,11,11a-octahydro-1,4b-methanobenzo[b]fluorene-3-carboxamide






