Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Counter-irritant.
Preparations
Camphorated Opium Tincture
Concentrated Camphorated Opium Tincture Concentrated Camphor Water
DEFINITION
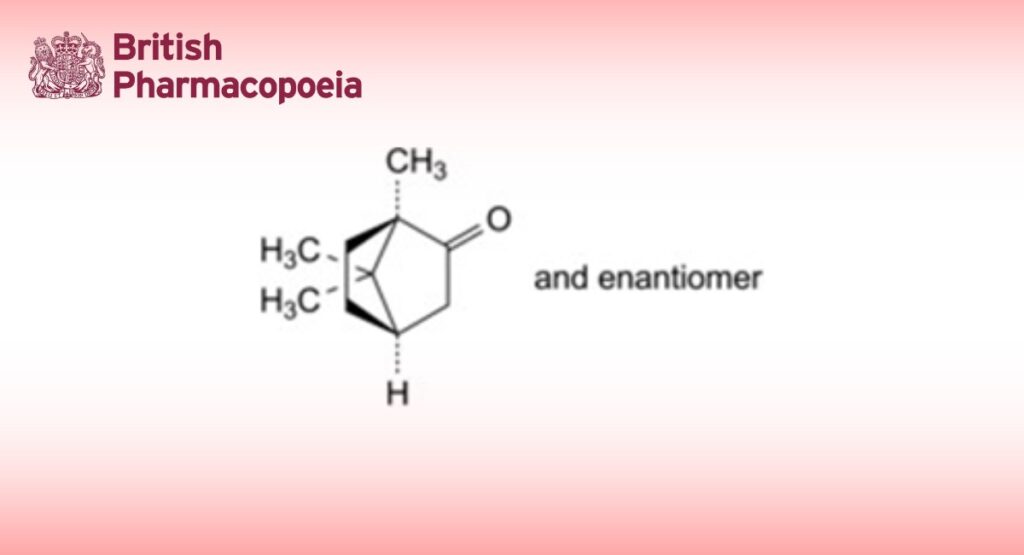
(1RS,4RS)-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-one.
CHARACTERS
Appearance
White or almost white, crystalline powder or friable, crystalline masses, highly volatile even at room temperature.
Solubility
Slightly soluble in water, very soluble in ethanol (96 per cent) and in light petroleum, freely soluble in fatty oils, very slightly soluble in glycerol.
IDENTIFICATION
First identification: A, C.
Second identification: A, B, D.
A. Optical rotation (see Tests).
B. Melting point (2.2.14): 172 °C to 180 °C.
C. Infrared absorption spectrophotometry (2.2.24).
Preparation Mulls in liquid paraffin R. Comparison racemic camphor CRS.
D. Dissolve 1.0 g in 30 mL of methanol R. Add 1.0 g of hydroxylamine hydrochloride R and 1.0 g of anhydrous sodium acetate R. Boil under a reflux condenser for 2 h. Allow to cool and add 100 mL of water R. A precipitate is formed. Filter, wash with 10 mL of water R and recrystallise from 10 mL of a mixture of 4 volumes of ethanol (96 per cent) R and 6 volumes of water R. The crystals, dried in vacuo, melt (2.2.14) at 118 °C to 121 °C.
TESTS
Carry out the weighings rapidly.
Solution S
Dissolve 2.50 g in 10 mL of ethanol (96 per cent) R and dilute to 25.0 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and colourless (2.2.2, Method II).
Acidity or alkalinity
Dissolve 1.0 g in 10 mL of ethanol (96 per cent) R and add 0.1 mL of phenolphthalein solution R1. The solution is colourless. Not more than 0.2 mL of 0.1 M sodium hydroxide is required to change the colour of the indicator.
Optical rotation (2.2.7)
-0.15° to + 0.15°, determined on solution S.
Related substances
Gas chromatography (2.2.28).
Test solution Dissolve 50 mg of the substance to be examined in hexane R and dilute to 50.0 mL with the same solvent.
Reference solution (a) Dissolve 50 mg of the substance to be examined and 50 mg of bornyl acetate R in hexane R and dilute to 50.0 mL with the same solvent.
Reference solution (b) Dilute 1.0 mL of the test solution to 200.0 mL with hexane R. Column:
— size: l = 2 m, Ø = 2 mm;
— stationary phase: diatomaceous earth for gas chromatography R impregnated with 10 per cent m/m of macrogol 20 000 R.
Carrier gas nitrogen for chromatography R. Flow rate 30 mL/min.
Temperature:
— column: 130 °C;
— injection port and detector: 200 °C.
Detection Flame ionisation.
Injection 1 µL.
Run time 3 times the retention time of camphor.
System suitability:
— resolution: minimum 1.5 between the peaks due to camphor and bornyl acetate in the chromatogram obtained with reference solution (a);
— signal-to-noise ratio: minimum 5 for the principal peak in the chromatogram obtained with reference solution (b).
Limits:
— any impurity: for each impurity, not more than 2 per cent of the area of the principal peak;
— total: not more than 4 per cent of the area of the principal peak;
— disregard limit: the area of the principal peak in the chromatogram obtained with reference solution (b).
Halogens
Maximum 100 ppm.
Dissolve 1.0 g in 10 mL of 2-propanol R in a distillation flask. Add 1.5 mL of dilute sodium hydroxide solution R and 50 mg of nickel-aluminium alloy R. Heat on a water-bath until the 2-propanol R has evaporated. Allow to cool and add 5 mL of water R. Mix and filter through a wet filter previously washed with water R until free from chlorides. Dilute the filtrate to 10.0 mL with water R. To 5.0 mL of this solution, add nitric acid R dropwise until the precipitate which forms is redissolved and dilute to 15 mL with water R. The solution complies with the limit test for chlorides (2.4.4).
Water
Dissolve 1 g in 10 mL of light petroleum R. The solution is clear (2.2.1).
Residue on evaporation
Maximum 0.05 per cent.
Evaporate 2.0 g on a water-bath and dry at 100-105 °C for 1 h. The residue weighs not more than 1 mg.