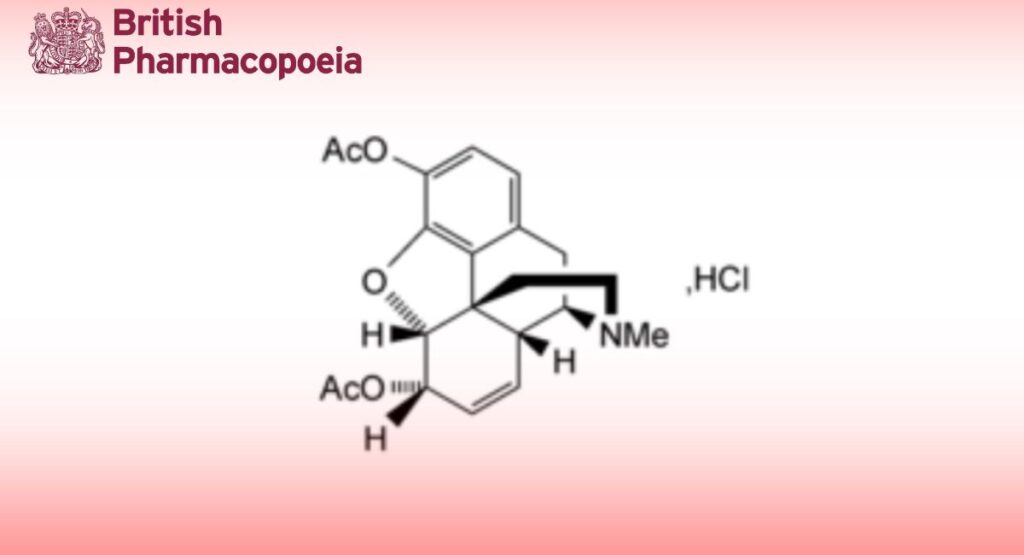
C21H23NO5,HCl,H2O 423.9 1502-95-0
Action and use
Opioid receptor agonist; analgesic.
Preparations
Bupivacaine and Diamorphine Injection
Diamorphine Tablets
Diamorphine Injection
DEFINITION
Diamorphine Hydrochloride is 4,5-epoxy-17-methylmorphinan-3,6-diyl diacetate hydrochloride monohydrate. It contains not less than 98.0% and not more than 102.0% of C21H23NO5,HCl, calculated with reference to the dried substance.
CHARACTERISTICS
A white or almost white, crystalline powder.
Freely soluble in water; soluble in ethanol (96%); practically insoluble in ether.
IDENTIFICATION
A. Dissolve a sufficient quantity in the minimum volume of dichloromethane and evaporate to dryness. The infrared absorption spectrum of the residue, Appendix II A, is concordant with the reference spectrum of diamorphine hydrochloride (RS 093).
B. Yields reaction A characteristic of chlorides, Appendix VI.
TESTS
Acidity
Dissolve 0.2 g in 10 mL of carbon dioxide-free water and titrate with 0.02M sodium hydroxide VS using methyl red solution as indicator. Not more than 0.2 mL of 0.02M sodium hydroxide VS is required.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) 0.5% w/v of the substance being examined in water.
(2) Dilute 1 volume of solution (1) to 50 volumes with water.
(3) A freshly prepared solution containing 0.1% w/v of the substance being examined in 0.01M sodium hydroxide.
(4) Dilute 1 volume of solution (2) to 20 volumes with water.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (12.5 cm × 4.6 mm) packed with base-deactivated octylsilyl silica gel for
chromatography, (5 μm) (Lichrospher RP-select B is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 283 nm.
(f) Inject 20 μL of each solution.
(g) Allow the chromatography to proceed for twice the retention time of the peak due to diamorphine hydrochloride.
MOBILE PHASE
0.11 % w/v of sodium octanesulfonate in a mixture of 10 volumes of glacial acetic acid, 10 volumes of methanol, 115 volumes of acetonitrile and 365 volumes of water.
SYSTEM SUITABILITY
The test is not valid unless: the chromatogram obtained with solution (3) exhibits two secondary peaks with retention times relative to the principal peak of about 0.23 (morphine) and 0.43 (6-O-acetyl-morphine); in the chromatogram obtained with solution (3), the resolution factor between the peaks due to morphine and 6-O-acetyl- morphine is at least 2.0.
LIMITS
In the chromatogram obtained with solution (1): the area of any peak corresponding to 6-O-acetylmorphine is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (2%); the sum of the areas of any other secondary peaks is not greater than 0.25 times the area of the principal peak in the chromatogram obtained with solution (2) (0.5%).
Disregard any peak with an area less than the area of the principal peak in the chromatogram obtained with solution (4) (0.1%).
Loss on drying
When dried to constant weight at 105°, loses 3.0 to 4.5% of its weight. Use 1 g.
Sulfated ash
Not more than 0.1%, Appendix IX A.
ASSAY
Dissolve 0.40 g in 50 mL of ethanol (96%) and add 5.0 mL of 0.01M hydrochloric acid VS. Titrate with 0.1M sodium hydroxide VS , determining the end point potentiometrically. Measure the volume of titrant required between the two points of inflection. Each mL of 0.1M sodium hydroxide VS is equivalent to 40.59 mg of C21H23NO5,HCl.
STORAGE
Diamorphine Hydrochloride should be protected from light.
IMPURITIES
The impurity limited by this monograph is:
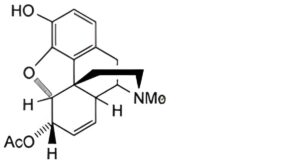
A. 6-O-acetylmorphine.