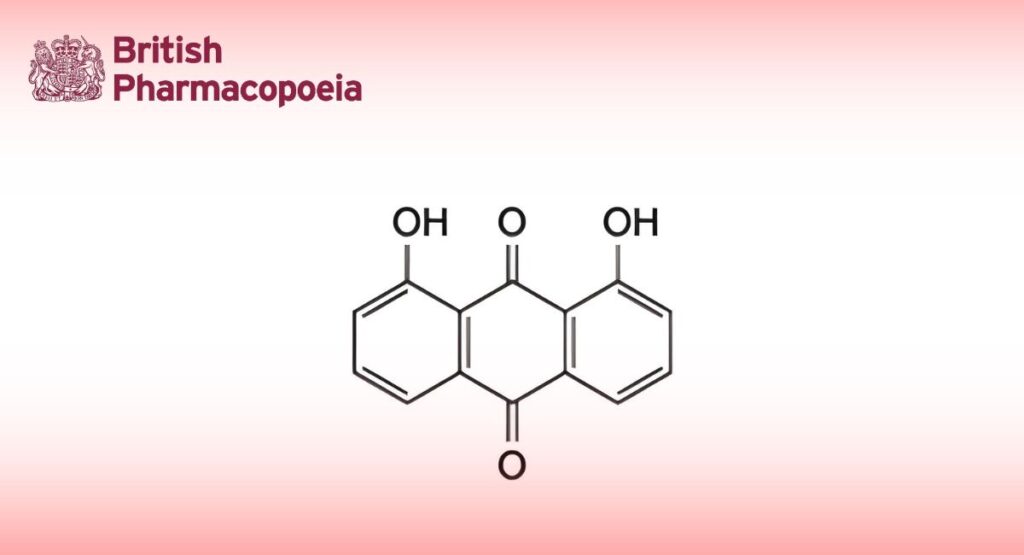
C14H8O4 240.2 117-10-2
Action and use
Anthraquinone stimulant laxative.
Preparation
Co-danthrusate Capsules
DEFINITION
Dantron is mainly 1,8-dihydroxyanthraquinone. It contains not less than 98.0% and not more than 102.0% of total phenols, calculated as C14H8O4 and with reference to the dried substance.
CHARACTERISTICS
An orange, crystalline powder.
Practically insoluble in water; slightly soluble in ether; very slightly soluble in ethanol (96%). It dissolves in solutions of alkali hydroxides.
IDENTIFICATION
A. The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of dantron (RS 083).
B. The light absorption, Appendix II B, in the range 230 to 350 nm of a 0.001% w/v solution in dichloromethane exhibits maxima at 255 nm and 285 nm and a less well-defined maximum at 275 nm. The absorbance at the maximum at 255 nm is about 0.82 and at the maximum at 285 nm is about 0.48, each calculated with reference to the dried substance.
C. Dissolve 5 mg in 5 mL of 1M sodium hydroxide. A clear red solution is produced immediately.
TESTS
Mercury
To 0.50 g in a Kjeldahl flask add 2.5 mL of nitric acid and allow to stand until the initial vigorous reaction has subsided. Add 2.5 mL of sulfuric acid and heat until dense white fumes are evolved. Cool, add 2.5 mL of nitric acid and heat until fumes are again evolved. Repeat the procedure with a further 2.5 mL of nitric acid, cool, add 50 mL of water, boil the solution until the volume has been reduced to about 25 mL and cool. Transfer to a separating funnel using water, dilute to about 50 mL with water and add 50 mL of 0.5M sulfuric acid. Add 100 mL of water, 2 g of hydroxylamine hydrochloride, 1 mL of 0.05M disodium edetate, 1 mL of glacial acetic acid and 5 mL of dichloromethane, shake, allow to separate and discard the dichloromethane layer. Titrate the aqueous layer with a 0.0008% w/v solution of dithizone in dichloromethane, shaking vigorously after each addition, allowing the layers to separate and discarding the dichloromethane layer, until the dichloromethane layer remains green. Repeat the operation using a solution prepared by diluting 1 mL of mercury standard solution (5 ppm Hg) to 100 mL with 0.5M sulfuric acid and beginning at the words ‘Add 100 mL of water…’. The volume of the dithizone solution required by the substance being examined does not exceed that required by the mercury standard solution.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Dissolve 50 mg of the substance being examined in 20 mL of tetrahydrofuran and dilute to 100 mL with the mobile phase.
(2) Dilute 1 volume of solution (1) to 50 volumes with the mobile phase.
(3) Dissolve 50 mg of dantron impurity standard BPCRS in 20 mL of tetrahydrofuran and dilute to 100 mL with the mobile phase.
CHROMATOGRAPHIC CONDITIONS
a) Use a stainless steel column (25 cm x 4.6 mm) packed with octadecylsilyl silica gel for chromatography (5 μm) (Nucleosil C18 is suitable).
(b) Use an isocratic system using the mobile phase described below.
(c) Use a flow rate of 1 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 254 nm.
(f) Inject 20 μL of each solution.
(g) Allow the chromatography to proceed for 1.5 times the retention time of the principal peak.
MOBILE PHASE
A mixture of 2.5 volumes of glacial acetic acid, 40 volumes of tetrahydrofuran and 60 volumes of water.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (3):
— — a peak due to 1-hydroxyanthraquinone appears immediately before the principal peak, as indicated in the reference chromatogram supplied with dantron impurity standard BPCRS;
— the height of the trough separating the two peaks is not greater than one third of the height of the peak due to 1-hydroxyanthraquinone.
LIMITS
In the chromatogram obtained with solution (1):
— the area of any peak corresponding to 1-hydroxyanthraquinone is not greater than 2.5 times the area of the principal peak in the chromatogram obtained with solution (2) (3.3% taking into account the correction factor of the impurity);
— — the sum of the areas of any other secondary peaks is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (2%);
— disregard any peak with a retention time less than one third of that of the principal peak.
Loss on drying
When dried to constant weight at 105°, loses not more than 0.5% of its weight. Use 1 g.
ASSAY
Dissolve 0.2 g in 50 mL of anhydrous pyridine and carry out Method II for non-aqueous titration, Appendix VIII A, using 0.1 M tetrabutylammonium hydroxide VS as titrant and determining the end point potentiometrically. Each mL of 0.1M tetrabutylammonium hydroxide VS is equivalent to 24.02 mg of total phenols, calculated as C14H8O4.