Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Penicillin antibacterial.
Preparations
Cloxacillin Benzathine Intramammary Infusion (Dry Cow)
Ampicillin Trihydrate and Cloxacillin Benzathine Intramammary Infusion (Dry Cow)
DEFINITION
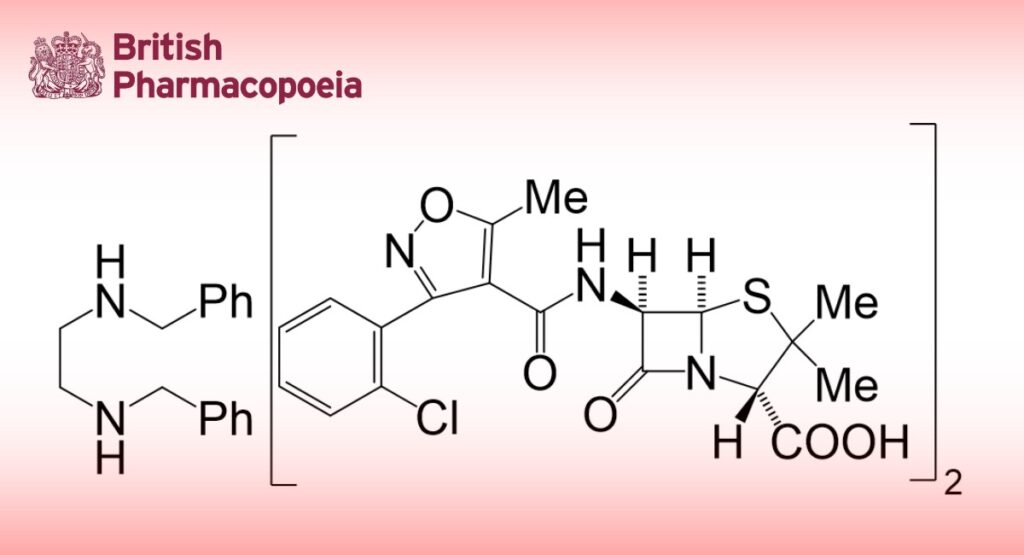
Cloxacillin Benzathine is N,N′-dibenzylethylenediammonium bis[(6R)-6-(3-o-chlorophenyl-5-methylisoxazole-4- carboxamido)penicillanate]. It contains not less than 92.0% of C16H20N2,(C19H18ClN3O5S)2 and not less than 20.0% and not more than 22.0% of benzathine, C16H20N2, each calculated with reference to the anhydrous substance.
CHARACTERISTICS
A white or almost white powder.
Slightly soluble in water; freely soluble in methanol; slightly soluble in ethanol (96%) and in propan-2-ol.
IDENTIFICATION
A. The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of cloxacillin benzathine (RSV 12).
B. Shake 0.1 g with 1 mL of 1M sodium hydroxide for 2 minutes, add 2 mL of ether, shake for 1 minute and allow to separate. Evaporate 1 mL of the ether layer to dryness, dissolve the residue in 2 mL of glacial acetic acid and add 1 mL of dilute potassium dichromate solution. A golden yellow precipitate is produced.
C. Shake 50 mg with 10 mL of water and filter. To 5 mL of the filtrate add a few drops of silver nitrate solution. No precipitate is produced. Heat 50 mg with 2 mL of alcoholic potassium hydroxide solution on a water bath for 15 minutes, add 15 mg of activated charcoal, shake and filter. Acidify the filtrate with 2M nitric acid. The solution yields reaction A characteristic of chlorides, Appendix VI.
TESTS
Water
Not more than 5.0% w/w, Appendix IX C. Use 0.5 g.
ASSAY
For cloxacillin benzathine
To 60 mg add 40 mL of methanol, shake to dissolve, add 25 mL of 1M sodium hydroxide and allow to stand for 30 minutes. Add 27.5 mL of 1M hydrochloric acid and sufficient water to produce 100 mL, mix, transfer 20 mL of the solution to a stoppered flask, add 30 mL of 0.01M iodine VS, close the flask with a wet stopper and allow to stand for 15 minutes protected from light. Titrate the excess of iodine with 0.02M sodium thiosulfate VS, using starch mucilage, added towards the end of the titration, as indicator. Add a further 12 mg of the substance being examined to 10 mL of water, swirl to disperse, add 30 mL of 0.01M iodine VS and titrate immediately with 0.02M sodium thiosulfate VS, using starch mucilage, added towards the end of the titration, as indicator. The difference between the titrations represents the volume of 0.01M iodine VS equivalent to the total penicillins present. Calculate the content of C16H20N2,(C19H18ClN3O5S)2 from the difference obtained by carrying out the assay simultaneously using cloxacillin benzathine BPCRS and from the declared content of C16H20N2,(C19H18ClN3O5S)2 in cloxacillin benzathine BPCRS.
For benzathine
To 1 g add 30 mL of a saturated solution of sodium chloride and 10 mL of 5M sodium hydroxide, shake well, and extract with four 50 mL quantities of ether. Wash the combined extracts with three 10 mL quantities of water, extract the combined washings with 25 mL of ether and add the extract to the main ether solution. Evaporate the ether solution to low bulk, add 2 mL of absolute ethanol and evaporate to dryness. To the residue add 50 mL of anhydrous acetic acid and titrate with 0.1 M perchloric acid VS, using 0.1 mL of 1-naphtholbenzein solution as indicator. Repeat the operation without the substance being examined. The difference between the titrations represents the amount of perchloric acid required to neutralise the liberated base. Each mL of 0.1M perchloric acid VS is equivalent to 12.02 mg of C16H20N2.
STORAGE
Cloxacillin Benzathine should be kept in an airtight container. If the material is sterile, the container should be sterile, tamper-evident and sealed so as to exclude micro-organisms.
LABELLING
The label states, where applicable, that the material is sterile.
Cloxacillin Benzathine intended for use in the manufacturer of either a parenteral dosage form or an intramammary infusion without a further appropriate sterilisation procedure complies with the following additional requirement.
Sterility
Complies with the test for sterility, Appendix XVI A.