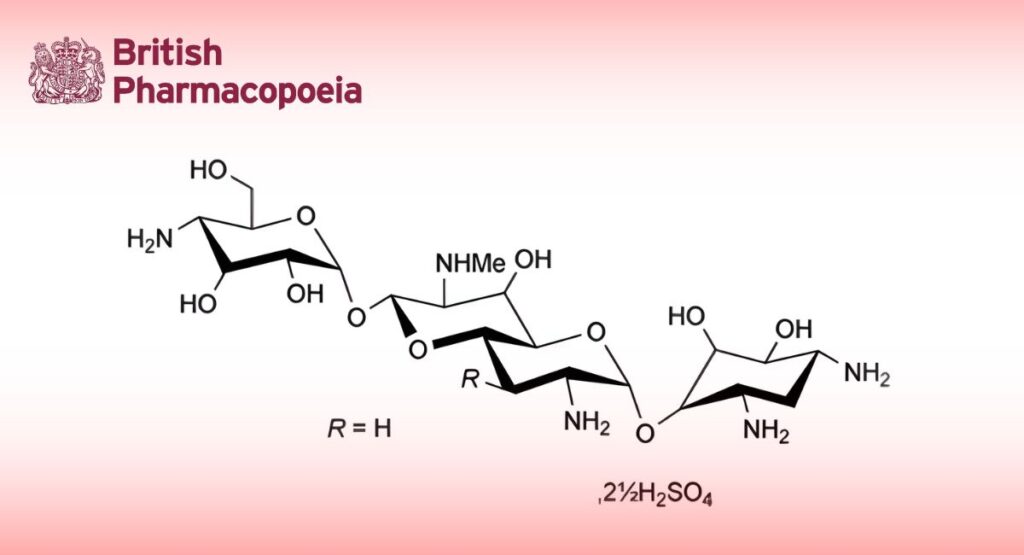
C21H41N5O11,21⁄2H2SO4 784.8 41194-16-5
Action and use
Aminoglycoside antibacterial.
Preparations
Apramycin Veterinary Oral Powder
Apramycin Premix
DEFINITION
Apramycin Sulfate is the sulfate of 4-O-[(2R,3R,4aS,6R,7S,8R,8aR)-3-amino-6-(4-amino-4-deoxy-α-D-glucopyranosyloxy)-8-hydroxy-7-methylaminoperhydropyrano[3,2-b]pyran-2-yl]-2-deoxystreptamine. It is produced by the growth of certain strains of Streptomyces tenebrarius or obtained by any other means. The potency is not less than 450 Units per mg, calculated with reference to the anhydrous substance.
CHARACTERISTICS
A light brown powder or granular material; hygroscopic. Freely soluble in water; practically insoluble in acetone, in ethanol (96%), in ether and in methanol.
IDENTIFICATION
A. Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions in water.
(1) 0.1% w/v of the substance being examined.
(2) 0.07% w/v of apramycin BPCRS.
(3) 0.07% w/v of each of apramycin BPCRS and tobramycin BPCRS.
Apramycin Sulfate
CHROMATOGRAPHIC CONDITIONS (a) Use a silica gel F254 precoated plate (Merck silica gel 60 F254 plates are suitable).
(b) Use the mobile phase as described below.
(c) Apply 5 μL of each solution.
(d) Develop the plate to 10 cm.
(e) After removal of the plate, allow it to dry in a current of warm air, spray with a mixture of equal volumes of a 46% w/v solution of sulfuric acid and a 0.2% w/v solution of naphthalene-1,3-diol in ethanol (96%) and heat at 150° for 5 to 10 minutes.
MOBILE PHASE
20 volumes of chloroform, 40 volumes of 13.5M ammonia and 60 volumes of methanol, equilibrated for 1 hour before use.
SYSTEM SUITABILITY
The test is not valid unless the chromatogram obtained with solution (3) shows two clearly separated principal spots.
CONFIRMATION
The principal spot in the chromatogram obtained with solution (1) corresponds in colour and position to that in the chromatogram obtained with solution (2).
B. In the test for Related substances, the retention time of the principal peak in the chromatogram obtained with solution
(1) corresponds to that of the principal peak in the chromatogram obtained with solution (2).
C. Yields reaction A characteristic of sulfates, Appendix VI.
TESTS
Sulfate
26.0 to 33.0% of SO4, calculated with reference to the anhydrous substance, when determined by the following method.
Dissolve 0.25 g in 100 mL of water, adjust to pH 11 with 13.5M ammonia and add 10 mL of 0.1M barium chloride VS. Titrate with 0.1M disodium edetate VS using 0.5 mg of phthalein purple as indicator; add 50 mL of ethanol (96%) when the colour of the solution begins to change and continue the titration until the violet-blue colour disappears. Each mL of 0.1M barium chloride VS is equivalent to 9.606 mg of SO4.
Caerulomycin and dipyridyl derivatives
Dissolve 0.5 g in water in a 100 mL graduated flask, add 10 mL of methanol and dilute to 100 mL with water. Place 5 mL in a 25 mL graduated flask and add 5 mL of an acetate buffer prepared by dissolving 8.3 g of anhydrous sodium acetate in 25 mL of water, adding 12 mL of glacial acetic acid and diluting to 100 mL with water. Mix, add 1 mL of a 10% w/v solution of hydroxylamine hydrochloride in water and mix again. Add 5 mL of a 1% w/v solution of ammonium iron(II) sulfate and dilute to 25 mL with water. Measure the absorbance of the resulting solution at 520 nm, Appendix II B, using in the reference cell a solution obtained by carrying out the same procedure without the substance being examined. The absorbance is not greater than that obtained by repeating the test using 5 mg of 2,2′-dipyridyl dissolved in 10 mL of methanol and diluted to 100 mL with water and beginning at the words ‘Place 5 mL in a 25 mL graduated flask…’ (1%).
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions in water.
(1) 0.50% w/v of the substance being examined.
(2) 0.35% w/v of apramycin BPCRS.
(3) Dilute 1 volume of solution (1) to 20 volumes.
(4) Dilute 1 volume of solution (3) to 50 volumes.
CHROMATOGRAPHIC CONDITIONS
(a) Use a column (25 cm × 4 mm) packed with fast cation-exchange polymeric beads (13 μm) with sulfonic acid functional groups (Dionex Fast Cation-1 is suitable) and a stainless steel post-column reaction coil (380 cm × 0.4 mm) with internal baffles. Use in the reaction coil ninhydrin reagent I at a flow rate approximately the same as that for the mobile phase.
(b) Use gradient elution and the mobile phase described below.
(c) Use a flow rate of 0.8 mL per minute.
(d) Use a column temperature of 130°. Maintain the post-column reaction coil at the same temperature.
(e) Use a detection wavelength of 568 nm.
(f) Inject 20 μL of each solution.
MOBILE PHASE
Mobile phase A A solution containing 1.961% w/v of sodium citrate, 0.08% v/v of liquefied phenol and 0.5% v/v of thiodiglycol, adjusted to pH 4.25 using hydrochloric acid.
Mobile phase B A solution containing 4.09% w/v of sodium chloride and 3.922% w/v of sodium citrate with 0.08% v/v of liquefied phenol, adjusted to pH 7.4 with hydrochloric acid.
Equilibrate the column using a mixture containing 75% of mobile phase A and 25% of mobile phase B. After each injection elute for 3 minutes using the same mixture and then carry out a linear gradient elution for 6 minutes to 100% of mobile phase B. Elute for a further 21 minutes using 100% of mobile phase B, then step-wise re-equilibrate to a mixture of 75% of mobile phase A and 25% of mobile phase B and elute for at least 10 minutes.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (2), the resolution factor between the peaks due to compound A and 3-hydroxyapramycin, identified using the reference chromatogram supplied with apramycin BPCRS, is at least 0.8.
LIMITS
Multiply the areas of all the secondary peaks by 0.5. [NOTE: This is to ensure that the impurities are calculated relative to Apramycin Sulfate (which contains about 50% w/w of apramycin).]
In the chromatogram obtained with solution (1):
the areas of any peaks corresponding to 3-hydroxyapramycin, lividamine/2-deoxystreptamine (combined), compound A and compound B (identified using the reference chromatogram supplied with apramycin BPCRS) are not greater than 1.4, 1.0, 0.4 and 0.4 times respectively the area of the principal peak in the chromatogram obtained with solution (3) (7%, 5%, 2% and 2% respectively);
the area of any other secondary peak is not greater than 0.4 times the area of the principal peak in the chromatogram obtained with solution (3) (2%);
the sum of the areas of all the secondary peaks is not greater than 3 times the area of
the principal peak in the chromatogram obtained with solution (3) (15%). Disregard any peak with an area less than the area of the peak in the chromatogram obtained with solution (4) (0.1%).
Sulfated ash
Not more than 1.0%, Appendix IX A, Method II. Use 1 g.
Water
Not more than 14.0% w/w, Appendix IX C. Use 0.2 g and 20 mL of a mixture containing 1 volume of methanol and 2 volumes of formamide as the solvent. The solvent mixture must be prepared at least 12 hours before use and should be
stored in an airtight container.
ASSAY
Carry out the microbiological assay of antibiotics, Appendix XIV A, Method B. The precision of the assay is such that the fiducial limits of error are not less than 95% and not more than 105% of the estimated potency.
IMPURITIES
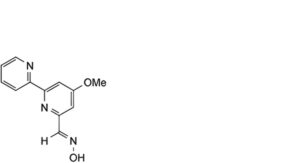
A. caerulomycin,
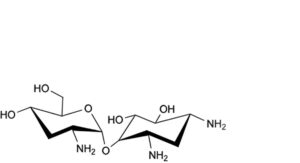
B. lividamine,
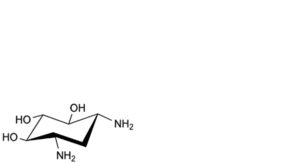
C. 2-deoxystreptamine,
D. 3-hydroxyapramycin; R = OH,
E. ‘compound A’,
F. ‘compound B’.