Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Dopamine receptor antagonist; neuroleptic.
Preparation
Zuclopenthixol Tablets
DEFINITION
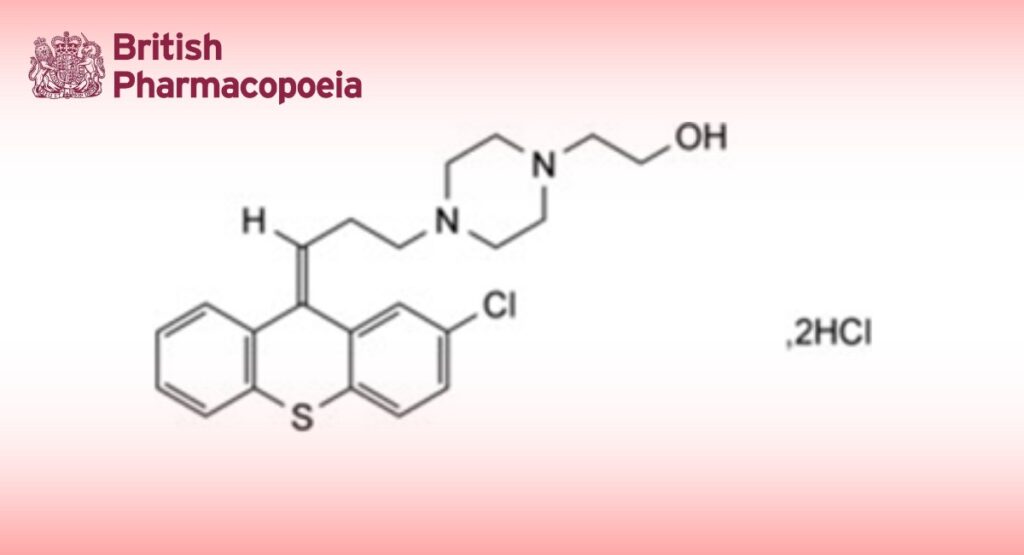
Zuclopenthixol Hydrochloride is (Z)-2-4-[3-(2-chlorothioxanthene-9-ylidene)propyl]piperazin-1-ylethanol dihydrochloride. It contains not less than 98.0% and not more than 101.0% of C22H25ClN2OS,2HCl, calculated with reference to the anhydrous substance.
CHARACTERISTICS
An off-white, granular powder.
Very soluble in water; sparingly soluble in ethanol (96%); very slightly soluble in ether.
IDENTIFICATION
A. The light absorption of a 0.0015% w/v solution in ethanol (96%), Appendix II B, in the range 210 to 350 nm exhibits two maxima at 230 and 268 nm. The absorbances at the maxima are about 1.0 and 0.4, respectively.
B. The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of zuclopenthixol hydrochloride ( RS 365).
C. Yields reaction A characteristic of chlorides, Appendix VI.
TESTS
Acidity
pH of a 1.0% w/v solution, 2.0 to 3.0, Appendix V L.
Free amine
Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions protected from light.
Solvent A Shake 0.6 mL of diethylamine with 150 mL of ethanol (96%) and add sufficient ethanol (96%) to produce 200 mL.
(1) 0.25% w/v solution of the substance being examined in solvent A.
(2) Dilute 1 volume of solution (1) to 400 volumes with solvent A.
CHROMATOGRAPHIC CONDITIONS
(a) Use a TLC silica gel F254 plate (Merck plates are suitable).
(b) Use the mobile phase as described below.
(c) Apply 4 µL of each solution.
(d) Develop the plate to 10 cm.
(e) After removal of the plate, allow it to dry in air, spray with a mixture of equal volumes of sulfuric acid and absolute ethanol, heat at 110° for 5 minutes and examine under ultraviolet light (365 nm) immediately.
MOBILE PHASE
2 volumes of water, 10 volumes of 13.5M ammonia, 20 volumes of butan-1-ol and 65 volumes of acetone.
LIMITS
In the chromatogram obtained with solution (1) any secondary spot of the same colour and at an Rf value lower than that of the principal spot is not more intense than the spot in the chromatogram obtained solution (2) (0.25%).
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions protected from light.
Solution A 18 volumes of acetonitrile and 82 volumes of water.
(1) Dissolve 20 mg of the substance being examined in 200 mL of solution A.
(2) Dilute 1 volume of solution (1) to 100 volumes with solution A and further dilute 1 volume of this solution to 10 volumes with solution A.
(3) Dilute 3 volumes of solution (1) to 100 volumes with solution A.
(4) 0.002% w/v each of zuclopenthixol hydrochloride BPCRS and trans-clopenthixol hydrochloride BPCRS (impurity B) in solution A.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (5 cm × 2.1 mm) packed with octadecylsilyl silica gel for chromatography (3.5 µm) (Symmetry Shield RP18 is suitable).
(b) Use gradient elution and the mobile phase described below.
(c) Use a flow rate of 0.6 mL per minute.
(d) Use a column temperature 40°.
(e) Use a detection wavelength of 270 nm.
(f) Inject 5 µL of each solution.
MOBILE PHASE
Mobile phase A 1 volume of trifluoroacetic acid and 2500 volumes of water.
Mobile phase B 1 volume of trifluoroacetic acid and 25 volumes of acetonitrile.
Mobile phase C methanol.
| Time (Minutes) | Mobile phase A (% v/v) | Mobile phase B (% v/v) | Mobile phase C (% v/v) | Comment |
| 0-8 | 82 | 18 | 0 | isocratic |
| 8-17 | 82→5 | 18→10 | 0→85 | linear gradient |
| 17-19 | 5→82 | 10→18 | 85→0 | linear gradient |
| 19-30 | 82 | 18 | 0 | re-equilibration |
When the chromatograms are recorded under the prescribed conditions, the retention times relative to zuclopenthixol (retention time about 6 minutes) are: impurity A, about 0.1; impurity B, about 1.2.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (4), the resolution between the peaks corresponding to zuclopenthixol and impurity B is at least 2.0.
LIMITS
In the chromatogram obtained with solution (1):
The area of any peak due to trans-zuclopenthixol is not greater than the area of the principal peak in the chromatogram obtained with solution (3) (3%);
The area of any other secondary peak is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.1%).
The sum of the areas of any secondary peak, excluding trans-clopenthixol hydrochloride, is not greater than five times the area of the principal peak in the chromatogram obtained with solution (2) (0.5%).
Disregard any peak with an area less than half the area of the principal peak in the chromatogram obtained with solution (2) (0.05%).
Water
Not more than 2.5%, determined on 0.5 g, Appendix IX C.
Sulfated ash
Not more than 0.1%, Appendix IX A.
ASSAY
Dissolve 0.5 g in 50 mL of anhydrous acetic acid and carry out Method I for non-aqueous titration, Appendix VIII A, using crystal violet solution as indicator. Each mL of 0.1M perchloric acid VS is equivalent to 23.70 mg of C22H25ClN2OS,2HCl.
STORAGE
Zuclopenthixol Hydrochloride should be protected from light.
IMPURITIES
A. 2-chloro-9-(1-hydroxy-3-(4-(2-hydroxyethyl)]piperazin-1-ylpropyl)thioxanthen9-ol,
B. trans-clopenthixol,
C. 2-chlorothioxanthone,
D. 9-allyl-2-chlorothioxanthen-9-ol,
E. 9-allylidene-2-chlorothioxanthene,
F. 2-chloro-9-(3-piperazin-1-ylpropylidene)thioxanthene.