Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Dopamine receptor antagonist; neuroleptic.
Preparation
Zuclopenthixol Decanoate Injection
DEFINITION
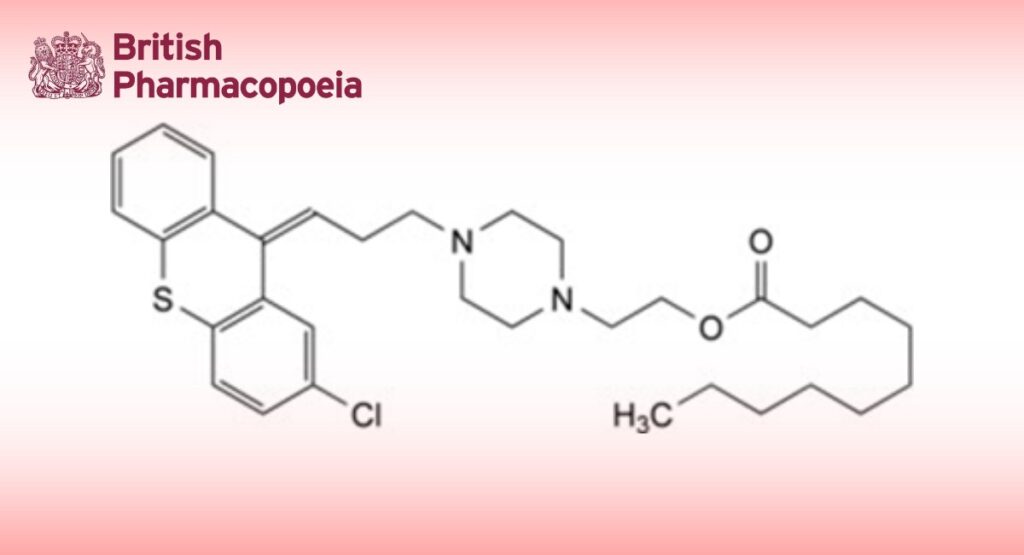
2-[4-[3-[(9Z)-2-Chloro-9H-thioxanthen-9-ylidene]propyl]piperazin-1-yl]ethyl decanoate.
Content
98.0 per cent to 102.0 per cent (dried substance).
CHARACTERS
Appearance
Yellow, viscous, oily liquid.
Solubility
Very slightly soluble in water, very soluble in ethanol (96 per cent) and in methylene chloride.
IDENTIFICATION
Infrared absorption spectrophotometry (2.2.24).
Comparison Ph. Eur. reference spectrum of zuclopenthixol decanoate.
TESTS
Appearance of solution
The solution is clear (2.2.1).
Using an ultrasonic bath, dissolve 1.0 g in ethanol (96 per cent) R and dilute to 20.0 mL with the same solvent.
Related substances
Liquid chromatography (2.2.29). Carry out the test protected from light and prepare the solutions immediately before use.
Solution A Dissolve 8.89 g of docusate sodium R in water for chromatography R, stirring for about 6-8 h, and dilute to 1000 mL with the same solvent.
Test solution Dissolve 25.0 mg of the substance to be examined in acetonitrile R and dilute to 100.0 mL with the same solvent.
Reference solution (a) Dilute 1.0 mL of the test solution to 100.0 mL with acetonitrile R.
Reference solution (b) Dissolve 5.0 mg of zuclopenthixol impurity B CRS in acetonitrile R and dilute to 100.0 mL with the same solvent. Dilute 5.0 mL of this solution to 100.0 mL with acetonitrile R.
Reference solution (c) Dissolve the contents of a vial of zuclopenthixol for system suitability CRS (containing impurities A, B and C) in 1 mL of methanol R.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 µm);
— temperature: 40 °C.
Mobile phase Mix 25 volumes of solution A and 75 volumes of anhydrous ethanol R, then add 0.1 volumes of phosphoric acid R.
Flow rate 1.0 mL/min.
Detection Spectrophotometer at 270 nm.
Injection 20 µL.
Run time Twice the retention time of zuclopenthixol decanoate.
Identification of impurities Use the chromatogram supplied with zuclopenthixol for system suitability CRS and the chromatograms obtained with reference solutions (b) and (c) to identify the peaks due to impurities A, B and C.
Relative retention With reference to zuclopenthixol decanoate (retention time = about 12 min): impurity C = about 0.4; impurity B = about 0.5; impurity A = about 1.1.
System suitability Reference solution (c):
— peak-to-valley ratio: minimum 2.0, where Hp = height above the baseline of the peak due to impurity C and
Hv = height above the baseline of the lowest point of the curve separating this peak from the peak due to impurity B; and minimum 2.5, where Hp = height above the baseline of the peak due to impurity A and Hv = height above the baseline of the lowest point of the curve separating this peak from the peak due to zuclopenthixol decanoate.
Limits:
— impurity A: not more than 1.3 times the area of the principal peak in the chromatogram obtained with reference solution (a) (1.3 per cent);
— impurity B: not more than 0.2 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.2 per cent);
— impurity C: not more than 0.3 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.3 per cent);
— unspecified impurities: for each impurity, not more than 0.1 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.10 per cent);
— total: not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (1.5 per cent);
— disregard limit: 0.05 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.05 per cent).
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in vacuo at 60 °C for 3 h.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.250 g in 50 mL of anhydrous acetic acid R. Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M perchloric acid is equivalent to 27.76 mg of C32H43ClN2O2S.
STORAGE
Under an inert gas in an airtight container, protected from light, at -20 °C or below.
IMPURITIES
Specified impurities A, B, C.
A. 2-[4-[3-[(9E)-2-chloro-9H-thioxanthen-9-ylidene]propyl]piperazin-1-yl]ethyl decanoate,
B. 2-chloro-9H-thioxanthen-9-one,
C. 2-[4-[3-[(9Z)-2-chloro-9H-thioxanthen-9-ylidene]propyl]piperazin-1-yl]ethanol.