Action and use
Non-benzodiazepine hypnotic.
DEFINITION
Zopiclone Tablets contain Zopiclone.
The tablets comply with the requirements stated under Tablets and with the following requirements.
Content of zopiclone, C17H17ClN6O3
95.0 to 105.0% of the stated amount.
Carry out all of the following procedures protected from light.
IDENTIFICATION
Shake a quantity of the powdered tablets containing 37.5 mg of Zopiclone with 30 mL of acetone, add sufficient acetone to produce 50 mL and filter. Evaporate the filtrate to dryness on a water bath and dry the residue in an oven at 60° for 2 hours. The infrared absorption spectrum of the dried residue, Appendix II A, is concordant with the reference spectrum of zopiclone (RS 430).
TESTS
Dissolution
Comply with the requirements for Monographs of the British Pharmacopoeia in the dissolution test for tablets and capsules, Appendix XII B1.
TEST CONDITIONS
(a) Use Apparatus 2, rotating the paddle at 50 revolutions per minute.
(b) Use 500 mL of 0.1M hydrochloric acid, at a temperature of 37°, as the dissolution medium.
PROCEDURE
(1) After 45 minutes withdraw a sample of the medium and measure the absorbance of the filtered sample, suitably diluted with the dissolution medium if necessary, at the maximum at 305 nm, Appendix II B using 0.1M hydrochloric acid in the reference cell.
(2) Measure the absorbance of a suitable solution of zopiclone BPCRS using 0.1M hydrochloric acid in the reference cell.
DETERMINATION OF CONTENT
Calculate the total content of zopiclone, C17H17ClN6O3, in the medium using the declared content of C17H17ClN6O3 in zopiclone BPCRS.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Mix with the aid of ultrasound a quantity of the powdered tablets containing 37.5 mg of Zopiclone with 50 mL of the mobile phase for 30 minutes, shake for a further 20 minutes. Cool the solution and add sufficient of the mobile phase to produce 100 mL, mix and filter through a 0.45-μm PTFE filter.
(2) Dilute 1 volume of solution (1) to 100 volumes with the mobile phase.
(3) 0.0006% w/v of 2-amino-5-chloropyridine BPCRS and 0.0001% w/v of each zopiclone BPCRS and zopiclone oxide EPCRS in the mobile phase.
(4) Dilute 1 volume of solution (2) to 10 volumes with the mobile phase.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (25 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (5 μm) (Symmetry C18 is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1.5 mL per minute.
(d) Use a detection wavelength of 303 nm.
(e) Use a column temperature of 30°.
(f) Inject 20 μL of each solution.
(g) Allow the chromatography to proceed for twice the retention time of zopiclone.
When the chromatograms are recorded under the prescribed conditions the retention times relative to zopiclone (retention time, about 26 minutes) are: 2-amino-5-chloropyridine, about 0.3 and zopiclone oxide, about 0.8.
MOBILE PHASE
375 volumes of acetonitrile and 625 volumes of a solution containing 0.5% w/v of sodium dodecyl sulfate and 0.01% w/v of sodium dihydrogen orthophosphate previously adjusted to pH 4.0 with orthophosphoric acid.
SYSTEM SUITABILITY
The test is not valid unless: in the chromatogram obtained with solution (3), the resolution between the peaks due to zopiclone and zopiclone-N-oxide is at least 3.0; in the chromatogram obtained with solution (4), the signal-to-noise ratio of the principal peak is not less than 10.
LIMITS
In the chromatogram obtained with solution (1):
the area of the peak due to 2-amino-5-chloropyridine is not greater than the area of the peak due to 2-amino-5-chloropyridine in solution (3) (1.5%);
excluding 2-amino-5-chloropyridine, the area of any secondary peak is not greater than 0.3 times the area of the principal peak in the chromatogram obtained with solution (2) (0.3%);
the sum of the impurities is not greater than 2%.
Disregard any peak with an area less than the area of the principal peak in solution (4) (0.1%).
Uniformity of content
Tablets containing less than 2 mg and/or less than 2% w/w of Zopiclone comply with the requirements stated under Tablets using the following method of analysis.
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Finely crush one tablet, add sufficient of the mobile phase to produce a solution with an expected concentration of 0.0075% w/v, shake and filter.
(2) 0.0075% w/v of zopiclone BPCRS in the mobile phase.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (25 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (5 μm) (Symmetry C18 is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1.5 mL per minute.
(d) Use a detection wavelength of 303 nm.
(e) Use a column temperature of 30°.
(f) Inject 10 μL of each solution.
MOBILE PHASE
1 volume of acetonitrile and 1 volume of a solution containing 0.5% w/v of sodium dodecyl sulfate and 0.1% w/v of sodium dihydrogen orthophosphate.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (2), the symmetry factor of the peak due to zopiclone is at most 1.4.
DETERMINATION OF CONTENT
Calculate the content of C17H17ClN6O3 in the tablets using the declared content of C17H17ClN6O3 in zopiclone BPCRS.
ASSAY
For tablets containing less than 2 mg and/or less than 2% w/w of zopiclone
Use the average of the individual results determined in the test for Uniformity of content.
For tablets containing 2 mg or more and 2% w/w or more of zopiclone
Weigh and powder 20 tablets. Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Shake a quantity of the powdered tablets containing 37.5 mg of Zopiclone with 60 mL of the mobile phase for 20 minutes, add sufficient of the mobile phase to produce 500 mL, mix and filter (Whatman GF/C is suitable), discarding the first 10 mL of filtrate.
(2) 0.0075% w/v of zopiclone BPCRS in the mobile phase.
CHROMATOGRAPHIC CONDITIONS
The chromatographic conditions described under Uniformity of content may be used.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (2), the symmetry factor of the peak due to zopiclone is at most 1.4.
DETERMINATION OF CONTENT
Calculate the content of C17H17ClN6O3 in the tablets using the declared content of C17H17ClN6O3 in zopiclone BPCRS.
STORAGE
Zopiclone Tablets should be protected from light.
IMPURITIES
In addition to the impurities listed under Zopiclone, the impurities limited by the requirements of this monograph include:
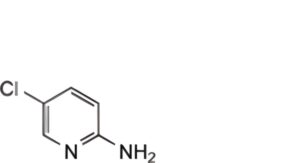
2-amino-5-chloropyridine.






