Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Dopamine D2 receptor antagonist; serotonin 5HT2 receptor antagonist; neuroleptic.
DEFINITION
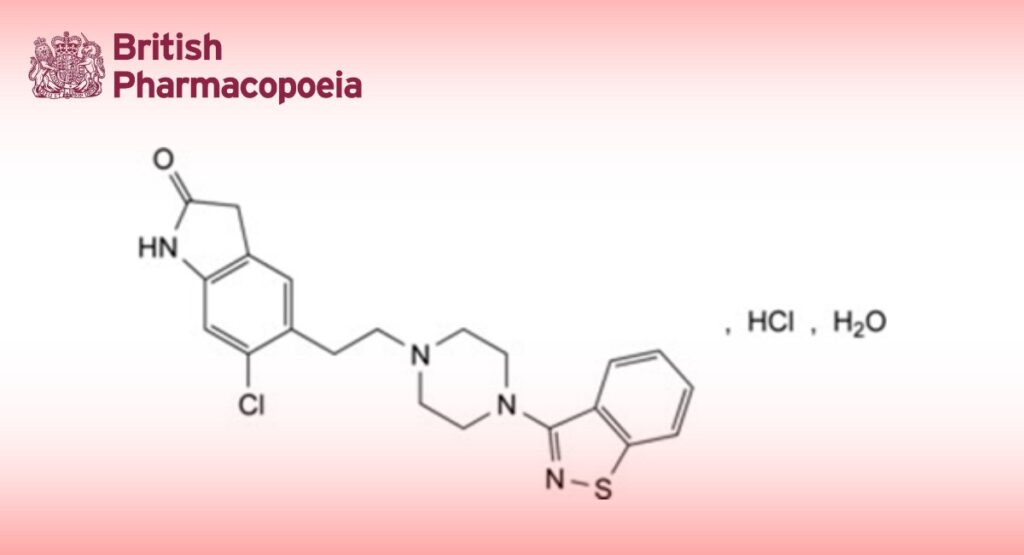
5-[2-[4-(1,2-Benzisothiazol-3-yl)piperazin-1-yl]ethyl]-6-chloro-1,3-dihydro-2H-indol-2-one hydrochloride monohydrate.
Content
98.0 per cent to 102.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or slightly pink powder.
Solubility
Practically insoluble in water, slightly soluble in methanol and in methylene chloride. It shows polymorphism (5.9).
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24).
Comparison ziprasidone hydrochloride monohydrate CRS.
If the spectra obtained in the solid state show differences, dissolve the substance to be examined and the reference substance separately in methanol R, evaporate to dryness and record new spectra using the residues.
B. Suspend 30 mg in 2 mL of water R, acidify with 0.15 mL of dilute nitric acid R and filter. The clear filtrate gives reaction (a) of chlorides (2.3.1).
TESTS
Related substances
Liquid chromatography (2.2.29). Carry out the test protected from light and prepare the solutions immediately before use. Solvent mixture A water R, methanol R (40:60 V/V).
Solvent mixture B hydrochloric acid R, water R, methanol R (0.04:20:80 V/V/V).
Test solution (a) Dissolve 23 mg of the substance to be examined in solvent mixture A and dilute to 100.0 mL with solvent mixture A.
Test solution (b) Dissolve 23 mg of the substance to be examined in solvent mixture B and dilute to 50.0 mL with solvent mixture B.
Reference solution (a) Dissolve 2.5 mg of ziprasidone for system suitability 1 CRS (containing impurities A, B and C) in solvent mixture A and dilute to 10.0 mL with solvent mixture A.
Reference solution (b) Dilute 1.0 mL of test solution (b) to 100.0 mL with solvent mixture B. Dilute 1.0 mL of this solution to 10.0 mL with solvent mixture B.
Reference solution (c) Dissolve the contents of a vial of ziprasidone for system suitability 2 CRS (containing impurities D and E) in 1.0 mL of solvent mixture B.
A. Column:
— size: l = 0.15 m, Ø = 4.6 mm;
— stationary phase: spherical octylsilyl silica gel for chromatography R (5 µm);
— temperature: 40 °C.
Mobile phase:
— mobile phase A: mix 40 volumes of methanol R and 60 volumes of a 6.8 g/L solution of potassium dihydrogen phosphate R previously adjusted to pH 2.5 with phosphoric acid R;
— mobile phase B: methanol R;
| Time (min) | Mobile phase A (per cent V/V) | Mobile phase B (per cent V/V) |
| 0 – 20 | 100 | 0 |
| 20 – 21 | 100 → 0 | 0 → 100 |
| 21 – 24 | 0 | 100 |
Flow rate 1.5 mL/min.
Detection Spectrophotometer at 229 nm.
Injection 20 µL of test solutions (a) and (b) and reference solutions (a) and (b).
Identification of impurities Use the chromatogram supplied with ziprasidone for system suitability 1 CRS and the chromatogram obtained with reference solution (a) to identify the peaks due to impurities A, B and C.
Relative retention With reference to ziprasidone (retention time = about 7 min): impurity A = about 0.4; impurity B = about 0.8; impurity C = about 0.9.
System suitability Reference solution (a):
— peak-to-valley ratio: minimum 1.2, where Hp = height above the baseline of the peak due to impurity C and
Hv = height above the baseline of the lowest point of the curve separating this peak from the peak due to impurity B.
Limits:
— correction factor: for the calculation of content, multiply the peak area of impurity A by 0.7;
— impurity B in test solution (b): not more than twice the area of the principal peak in the chromatogram obtained with reference solution (b) (0.2 per cent);
— impurity A in test solution (b): not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.15 per cent);
— impurity C in test solution (a): not more than 0.75 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.15 per cent);
— unspecified impurities in test solution (b): for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (0.10 per cent);
— disregard limit in test solution (b): 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 per cent); disregard the peak due to impurity C and any peak with a retention time greater than 20 min.
B. Column:
— size: l = 0.15 m, Ø = 4.6 mm;
— stationary phase: spherical octylsilyl silica gel for chromatography R (5 µm);
— temperature: 35 °C.
Mobile phase Mix 5 volumes of methanol R, 40 volumes of a 6.8 g/L solution of potassium dihydrogen phosphate R adjusted to pH 6.0 with a 280 g/L solution of potassium hydroxide R, and 55 volumes of acetonitrile R1. Flow rate 1.0 mL/min.
Detection Spectrophotometer at 229 nm.
Injection 20 µL of test solution (b) and reference solutions (b) and (c).
Run time 11 times the retention time of ziprasidone.
Identification of impurities Use the chromatogram supplied with ziprasidone for system suitability 2 CRS and the chromatogram obtained with reference solution (c) to identify the peaks due to impurities D and E.
Relative retention With reference to ziprasidone (retention time = about 4.5 min): impurity D = about 2.0; impurity E = about 3.0.
System suitability Reference solution (c):
— resolution: minimum 6.0 between the peaks due to ziprasidone and impurity D.
Limits:
— correction factors: for the calculation of content, multiply the peak areas of the following impurities by the corresponding correction factor: impurity D = 1.4; impurity E = 0.5;
— impurities D, E: for each impurity, not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.15 per cent);
— unspecified impurities: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (0.10 per cent);
— disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 per cent); disregard any peak eluting before the peak due to ziprasidone.
Limit:
— total for tests A and B: maximum 0.5 per cent.
Water (2.5.12)
3.7 per cent to 5.0 per cent, determined on 0.250 g.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Liquid chromatography (2.2.29). Carry out the test protected from light and prepare the solutions immediately before use. Solvent mixture water R, methanol R (40:60 V/V).
Test solution Dissolve 23.0 mg of the substance to be examined in the solvent mixture and dilute to 100.0 mL with the solvent mixture.
Reference solution Dissolve 23.0 mg of ziprasidone hydrochloride monohydrate CRS in the solvent mixture and dilute to 100.0 mL with the solvent mixture.
Column:
— size: l = 0.15 m, Ø = 4.6 mm;
— stationary phase: spherical octylsilyl silica gel for chromatography R (5 µm);
— temperature: 40 °C.
Mobile phase Mix 40 volumes of methanol R and 60 volumes of a 6.8 g/L solution of potassium dihydrogen phosphate R adjusted to pH 3.0 with phosphoric acid R.
Flow rate 1.5 mL/min.
Detection Spectrophotometer at 229 nm.
Injection 20 µL.
Run time Twice the retention time of ziprasidone.
Retention time Ziprasidone = about 7 min.
System suitability Reference solution:
— symmetry factor: maximum 2.0 for the peak due to ziprasidone.
Calculate the percentage content of C21H22Cl2N4OS from the declared content of ziprasidone hydrochloride monohydrate CRS.
STORAGE
Protected from light.
IMPURITIES
Specified impurities A, B, C, D, E.
A. 3-piperazin-1-yl-1,2-benzisothiazole,
B. 5-[2-[4-(1,2-benzisothiazol-3-yl)piperazin-1-yl]ethyl]-6-chloro-1H-indole-2,3-dione,
C. 2-[2-amino-5-[2-[4-(1,2-benzisothiazol-3-yl)piperazin-1-yl]ethyl]-4-chlorophenyl]acetic acid,
D. 5,5′-bis[2-[4-(1,2-benzisothiazol-3-yl)piperazin-1-yl]ethyl]-6,6′-dichloro-3-hydroxy-1,1′,3,3′-tetrahydro-2H,2′H-3,3′- biindole-2,2′-dione,
E. 3-(1,2-benzisothiazol-3-yl)-5-[2-[4-(1,2-benzisothiazol-3-yl)piperazin-1-yl]ethyl]-6-chloro-1,3-dihydro-2H-indol-2-one.