Edition: BP 2025 (Ph. Eur. 11.6 update)
Preparation
Paediatric Vitamins A, C and D Oral Drops
DEFINITION
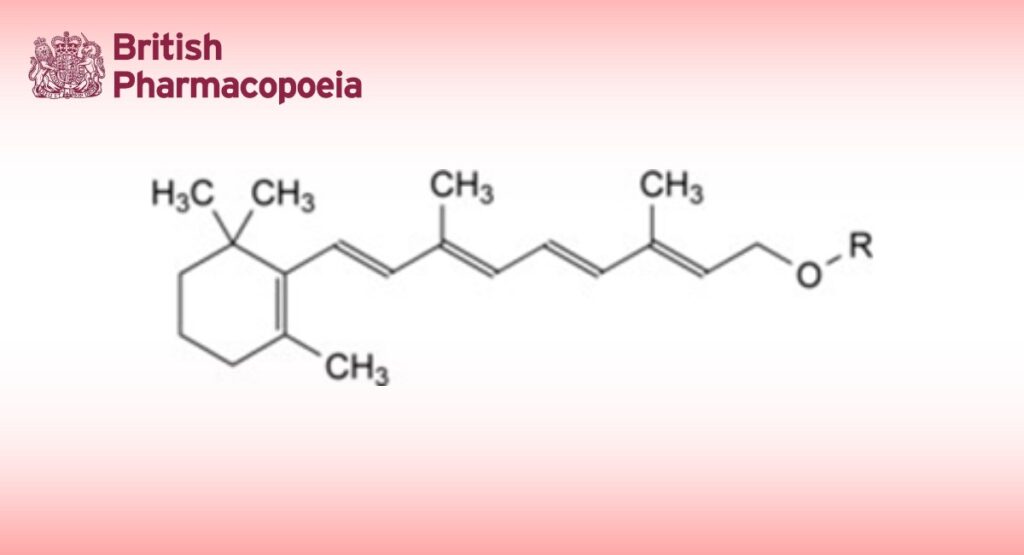
Vitamin A Refers to a number of substances of very similar structure (including (Z)-isomers) found in animal tissues and possessing similar activity. The principal and biologically most active substance is all-(E)-retinol (all-(E)-3,7-dimethyl-9- (2,6,6-trimethylcyclohex-1-enyl)nona-2,4,6,8-tetraen-1-ol; C20H30O). Vitamin A is generally used in the form of esters such as the acetate, propionate and palmitate.
Synthetic retinol ester Refers to an ester (acetate, propionate or palmitate) or a mixture of synthetic retinol esters.
The activity of vitamin A is expressed in retinol equivalents (R.E.). 1 mg R.E. corresponds to the activity of 1 mg of all-(E)- retinol. The activity of the other retinol esters is calculated stoichiometrically, so that 1 mg R.E. of vitamin A corresponds to the activity of:
— 1.147 mg of all-(E)-retinol acetate,
— 1.195 mg of all-(E)-retinol propionate,
— 1.832 mg of all-(E)-retinol palmitate.
International Units (IU) are also used to express the activity of vitamin A. 1 IU of vitamin A is equivalent to the activity of 0.300 µg of all-(E)-retinol. The activity of the other retinol esters is calculated stoichiometrically, so that 1 IU of vitamin A is equivalent to the activity of:
— 0.344 µg of all-(E)-retinol acetate,
— 0.359 µg of all-(E)-retinol propionate,
— 0.550 µg of all-(E)-retinol palmitate,
1 mg of retinol equivalent is equivalent to 3333 IU.
CHARACTERS
Appearance
Retinol acetate: pale-yellow crystals (mp: about 60 °C). Once melted retinol acetate tends to yield a supercooled melt. Retinol propionate: reddish-brown oily liquid.
Retinol palmitate: a fat-like, light yellow solid or a yellow oily liquid, if melted (mp: about 26 °C).
Solubility
All retinol esters are practically insoluble in water, soluble or partly soluble in anhydrous ethanol and miscible with organic solvents.
Vitamin A and its esters are very sensitive to the action of air, oxidising agents, acids, light and heat.
Carry out the assay and all tests as rapidly as possible, avoiding exposure to actinic light and air, oxidising agents, oxidation catalysts (e.g. copper, iron), acids and heat; use freshly prepared solutions.
IDENTIFICATION
A. Thin-layer chromatography (2.2.27).
Test solution Prepare a solution containing about 3.3 IU of vitamin A per microlitre in cyclohexane R containing 1 g/L of butylhydroxytoluene R.
Reference solution Prepare a 10 mg/mL solution of retinol esters CRS (i.e. 3.3 IU of each ester per microlitre) in cyclohexane R containing 1 g/L of butylhydroxytoluene R. Plate TLC silica gel F254 plate R.
Mobile phase ether R, cyclohexane R (20:80 V/V). Application 3 µL.
Development Over 2/3 of the plate.
Drying In air.
Detection Examine in ultraviolet light at 254 nm.
System suitability Reference solution:
— the chromatogram shows the individual spots of the corresponding esters. The elution order from bottom to top is: retinol acetate, retinol propionate and retinol palmitate.
Results The composition of esters is confirmed by the correspondence of the principal spot or spots of the test solution with those obtained with the reference solution.
B. Related substances (see Tests).
TESTS
Retinol
Thin-layer chromatography (2.2.27).
Test solution Prepare a solution in cyclohexane R, stabilised with a solution containing 1 g/L of butylhydroxytoluene R, containing about 330 IU of vitamin A per microlitre.
Reference solution Shake 1 mL of the test solution with 20 mL of 0.1 M tetrabutylammonium hydroxide in 2-propanol for 2 min and dilute to 100 mL with cyclohexane R, stabilised with a solution containg 1 g/L of butylhydroxytoluene R.
Plate TLC silica gel F254 plate R.
Mobile phase ether R, cyclohexane R (20:80 V/V). Application 3 µL.
Development Over a path of 15 cm.
Drying In air.
Detection Examine in ultraviolet light at 254 nm.
System suitability Reference solution:
— in the chromatogram obtained no or only traces of the retinol esters are seen.
Limit Any spot corresponding to retinol in the chromatogram obtained with the test solution is not more intense than the spot in the chromatogram obtained with the reference solution (1.0 per cent).
Related substances
Ultraviolet and visible absorption spectrophotometry (2.2.25).
Test solution The solution described under Activity.
Absorption maximum At 325 nm to 327 nm.
Absorbance ratios:
— A300/A326 = maximum 0.60;
— A350/A326 = maximum 0.54;
— A370/A326 = maximum 0.14.
The thresholds indicated under Related substances (Table 2034.-1) in the general monograph Substances for pharmaceutical use (2034) do not apply.
ACTIVITY
The activity of the substance is determined in order to be taken into account for the production of concentrates.
Dissolve 25-100 mg, weighed with an accuracy of 0.1 per cent, in 5 mL of pentane R and dilute with 2-propanol R1 to a presumed concentration of 10 IU/mL to 15 IU/mL. Measure the absorbance (2.2.25) at the absorption maximum at 326 nm. Calculate the activity of vitamin A in International Units per gram from the expression:
(A326×V ×1900)/(100×m)
A326 = absorbance at 326 nm,
m = mass of the substance to be examined, in grams,
V = total volume to which the substance to be examined is diluted to give 10 IU/mL to 15 IU/mL,
1900 = factor to convert the specific absorbance of esters of retinol into International Units per gram.
STORAGE
In an airtight container, protected from light.
Once the container has been opened, its contents are to be used as soon as possible; any part of the contents not used at once should be protected by an atmosphere of inert gas.
LABELLING
The label states:
— the number of International Units per gram,
— the name of the ester or esters.
IMPURITIES
A. R = H, CO-CH3: kitols (Diels-Alder dimers of vitamin A),
B. (3E,5E,7E)-3,7-dimethyl-9-[(1Z)-2,6,6-trimethylcyclohex-2-enylidene]nona-1,3,5,7-tetraene (anhydro-vitamin A),
C. (3E,5E,7E)-3,7-dimethyl-9-[(1Z)-2,6,6-trimethylcyclohex-2-enylidene]nona-3,5,7-trien-1-ol (retro-vitamin A),
D. oxidation products of vitamin A.