Vindesine Sulphate for Injection
Action and use
Vinca alkaloid cytotoxic.
DEFINITION
Vindesine Injection is a sterile solution of Vindesine Sulfate in a suitable liquid. It is prepared by dissolving Vindesine Sulfate for Injection in the requisite volume of a suitable diluent in accordance with the manufacturer’s instructions.
The injection complies with the requirements stated under Parenteral Preparations.
STORAGE
Vindesine Injection should be used immediately after preparation but, in any case, within the period recommended by the manufacturer when prepared and stored strictly in accordance with the manufacturer’s instructions.
VINDESINE SULFATE FOR INJECTION
DEFINITION
Vindesine Sulfate for Injection is a sterile material consisting of a mixture of one part by weight of Vindesine Sulfate and five parts by weight of a suitable carrier with or without other excipients. It is supplied in a sealed container.
The contents of the sealed container comply with the requirements for Powders for Injections or Infusions stated under Parenteral Preparations and with the following requirements.
Content of vindesine sulfate, C43H55N5O7,H2SO4
90.0 to 105.0% of the stated amount of anhydrous vindesine sulfate.
IDENTIFICATION
A. Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions in methanol.
(1) Dissolve a quantity of the contents of the injection in sufficient methanol to produce a solution containing the equivalent of 0.20% w/v of anhydrous vindesine sulfate.
(2) 0.20% w/v of vindesine sulfate EPCRS.
(3) 0.20% w/v each of vinblastine sulfate EPCRS, vincristine sulfate EPCRS and vindesine sulfate EPCRS.
CHROMATOGRAPHIC CONDITIONS
(a) Use as the coating silica gel GF254.
(b) Use the mobile phase as described below.
(c) Apply 10 μL of each solution.
(d) Develop the plate to 15 cm.
(e) After removal of the plate, dry in air and examine under ultraviolet light (254 nm).
MOBILE PHASE
5 volumes of diethylamine, 5 volumes of methanol and 90 volumes of chloroform.
SYSTEM SUITABILITY
The test is not valid unless the chromatogram obtained with solution (3) shows three clearly separated spots.
CONFIRMATION
The principal spot in the chromatogram obtained with solution (1) corresponds in position and colour to that in the chromatogram obtained with solution (2).
B. In the Assay, the principal peak in the chromatogram obtained with solution (1) has the same retention time as the principal peak in the chromatogram obtained with solution (2).
C. Yields the reactions characteristic of sulfates, Appendix VI.
TESTS
Clarity of solution
Dissolve the contents of a sealed container in 10 mL of carbon dioxide-free water. The solution is clear, Appendix IV A.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions in water. Keep the solutions in ice before use.
(1) To a quantity of injection add sufficient water to produce a solution expected to contain 0.10% w/v of anhydrous Vindesine Sulfate.
2) Dilute 1 volume of solution (1) to 50 volumes.
(3) Dissolve 1 mg of desacetylvinblastine EPCRS in water, add 1 mL of solution (1) and dilute to 50 mL.
(4) Dilute 1 volume of solution (2) to 20 volumes.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (15 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (5 μm) (Jones Apex ODS is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 2 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 270 nm.
(f) Inject 200 μL of each solution.
(g) Maintain the final concentration of the mobile phase until the total run time is twice the retention time of the principal peak in the chromatogram obtained with solution (1).
MOBILE PHASE
Mobile phase A: A 1.5% v/v solution of diethylamine adjusted to pH 7.4 with orthophosphoric acid.
Mobile phase B: methanol.
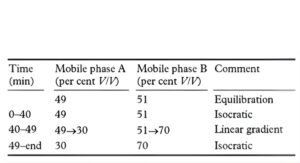
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (3):
the retention time of vindesine is less than 40 minutes;
the symmetry factor of the vindesine peak is not more than 2.0;
the resolution factor between the peaks due to vindesine and desacetylvinblastine is at least 2.0.
LIMITS
In the chromatogram obtained with the solution (1):
the area of any secondary peak is not greater than 1.5 times the area of the principal peak in the chromatogram obtained with solution (2) (3%);
the sum of the areas of any such peaks is not greater than 2.5 times the area of the principal peak in the chromatogram obtained with solution (2) (5%).
Disregard any peak with an area less than the area of the principal peak in the chromatogram obtained with solution (4) (0.1%).
Uniformity of content
The content of anhydrous vindesine sulfate in each of 10 individual containers as determined in the Assay is not less than 90.0% and not more than 110.0% of the average except that in one container the content may be not less than 80.0% and not more than 120.0% of the average.
ASSAY
Carry out the method for liquid chromatography, Appendix III D, using the following solutions. Keep the solutions in ice before use.
(1) Dissolve the contents of a sealed container in water and add sufficient water to produce a solution containing the equivalent of 0.05% w/v of anhydrous vindesine sulfate.
(2) Dissolve and dilute the entire contents of a vial of vindesine sulfate EPCRS with water to yield a concentration of approximately 0.05% w/v.
(3) Add 2 mL of solution (2) to 1 mg of desacetylvinblastine EPCRS.
CONDITIONS
(a) Use a stainless steel column (15 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (5 μm) (Jones Apex ODS is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 270 nm.
(f) Inject 20 μL of each solution.
MOBILE PHASE
38 volumes of a 1.5% v/v solution of diethylamine, previously adjusted to pH 7.4 with orthophosphoric acid, and 62 volumes of methanol.
SYSTEM SUITABILITY
The assay is not valid unless:
the resolution factor between the peaks corresponding to vindesine sulfate and desacetylvinblastine sulfate is greater than 1.5;
the symmetry factor of the vindesine peak is not more than 2.0.
DETERMINATION OF CONTENT
Calculate the content of vindesine sulfate, C43H55N5O7,H2SO4, in the container using the declared content of C43H55N5O7,H2SO4
in vindesine sulfate EPCRS. Repeat the procedure with a further nine containers. Calculate the average content of C43H55N5O7,H2SO4 per container from the 10 individual results obtained.
STORAGE
Vindesine Sulfate for Injection should be stored at a temperature of 2° to 8°.
IMPURITIES
The impurities limited by the requirements of this monograph include those listed in the monograph for Vindesine Sulfate.






