Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Vinca alkaloid cytotoxic.
Preparation
Vincristine Injection
DEFINITION
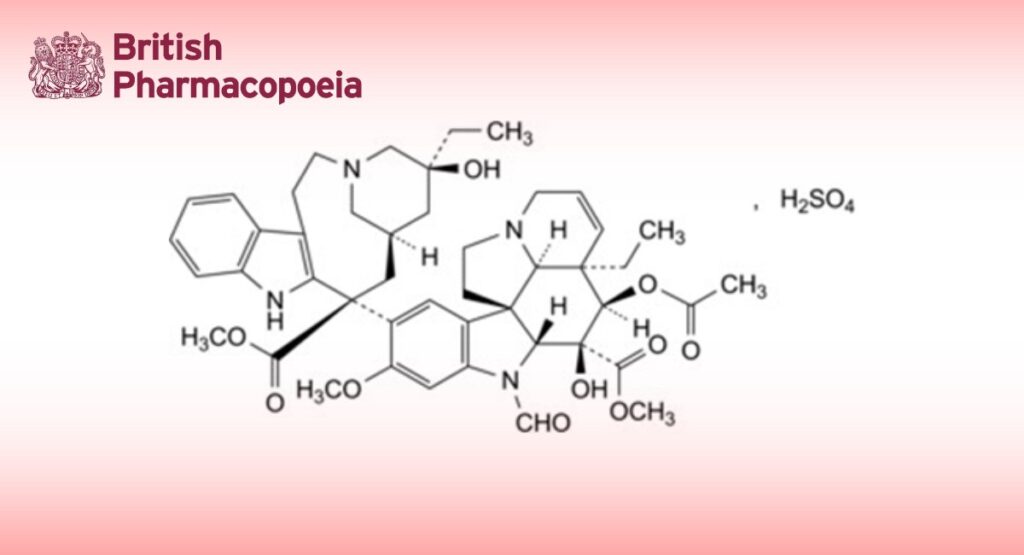
Methyl (3aR,4R,5S,5aR,10bR,13aR)-4-(acetyloxy)-3a-ethyl-9-[(5S,7R,9S)-5-ethyl-5-hydroxy-9-(methoxycarbonyl)-1,4,5,6,7,8,9,10-octahydro-2H-3,7-methanoazacycloundecino[5,4-b]indol-9-yl]-6-formyl-5-hydroxy-8- methoxy-3a,4,5,5a,6,11,12,13a-octahydro-1H-indolizino[8,1-cd]carbazole-5-carboxylate sulfate.
Content
95.0 per cent to 104.0 per cent (dried substance).
CHARACTERS
Appearance
White or slightly yellowish, crystalline powder, very hygroscopic.
Solubility
Freely soluble in water, slightly soluble in ethanol (96 per cent).
IDENTIFICATION
Infrared absorption spectrophotometry (2.2.24).
Comparison Ph. Eur. reference spectrum of vincristine sulfate.
TESTS
Solution S
Dissolve 50.0 mg in carbon dioxide-free water R and dilute to 10.0 mL with the same solvent. Keep the solution in iced water to carry out the test for related substances.
Appearance of solution
Solution S is clear (2.2.1) and not more intensely coloured than reference solution Y7 (2.2.2, Method I).
pH (2.2.3)
3.5 to 4.5.
Dilute 2 mL of solution S to 10 mL with carbon dioxide-free water R.
Related substances
Liquid chromatography (2.2.29). Keep the solutions in iced water before use. Test solution Dilute 1.0 mL of solution S to 5.0 mL with water R.
Reference solution (a) Dissolve the contents of a vial of vincristine sulfate CRS in 5.0 mL of water R to obtain a concentration of 1.0 mg/mL.
Reference solution (b) Dissolve 1.0 mg of vinblastine sulfate CRS in 1.0 mL of reference solution (a).
Reference solution (c) Dilute 1.0 mL of the test solution to 50.0 mL with water R. Reference solution (d) Dilute 1.0 mL of reference solution (c) to 20.0 mL with water R.
Precolumn:
— stationary phase: octylsilyl silica gel for chromatography R. Column:
— size: l = 0.25 m, Ø = 4.6 mm,
— stationary phase: octylsilyl silica gel for chromatography R (5 µm).
Mobile phase:
— mobile phase A: 1.5 per cent V/V solution of diethylamine R adjusted to pH 7.5 with phosphoric acid R,
— mobile phase B: methanol R,
| Time (min) | Mobile phase A (per cent V/V) | Mobile phase B (per cent V/V) |
| 0 – 12 | 38 | 62 |
| 12 – 27 | 38 → 8 | 62 → 92 |
Flow rate 2 mL/min.
Detection Spectrophotometer at 297 nm.
Injection 20 µL.
System suitability Reference solution (b):
— resolution: minimum 4 between the peaks due to vincristine and vinblastine.
Limits:
— any impurity: not more than the area of the principal peak in the chromatogram obtained with reference solution (c) (2.0 per cent),
— total: not more than 2.5 times the area of the principal peak in the chromatogram obtained with reference solution (c) (5.0 per cent),
— disregard limit: area of the peak in the chromatogram obtained with reference solution (d) (0.1 per cent).
Loss on drying
Maximum 12.0 per cent, determined on 3 mg by thermogravimetry (2.2.34). Heat the substance to be examined to 200 °C increasing the temperature by 5 °C/min, under a current of nitrogen for chromatography R, at a flow rate of 40 mL/min.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances, with the following modifications.
Mobile phase Mix 30 volumes of a 1.5 per cent V/V solution of diethylamine R adjusted to pH 7.5 with phosphoric acid R and 70 volumes of methanol R. Flow rate 1.0 mL/min.
Calculate the percentage content of C46H58N4O14S using the chromatogram obtained with reference solution (a) and the declared content of vincristine sulfate CRS.
STORAGE
In an airtight, glass container, protected from light, at a temperature not exceeding -20 °C. If the substance is sterile, store in a sterile, airtight, tamper-evident glass container.
IMPURITIES
Specified impurities A, B, C, D, H.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) E, F, G.
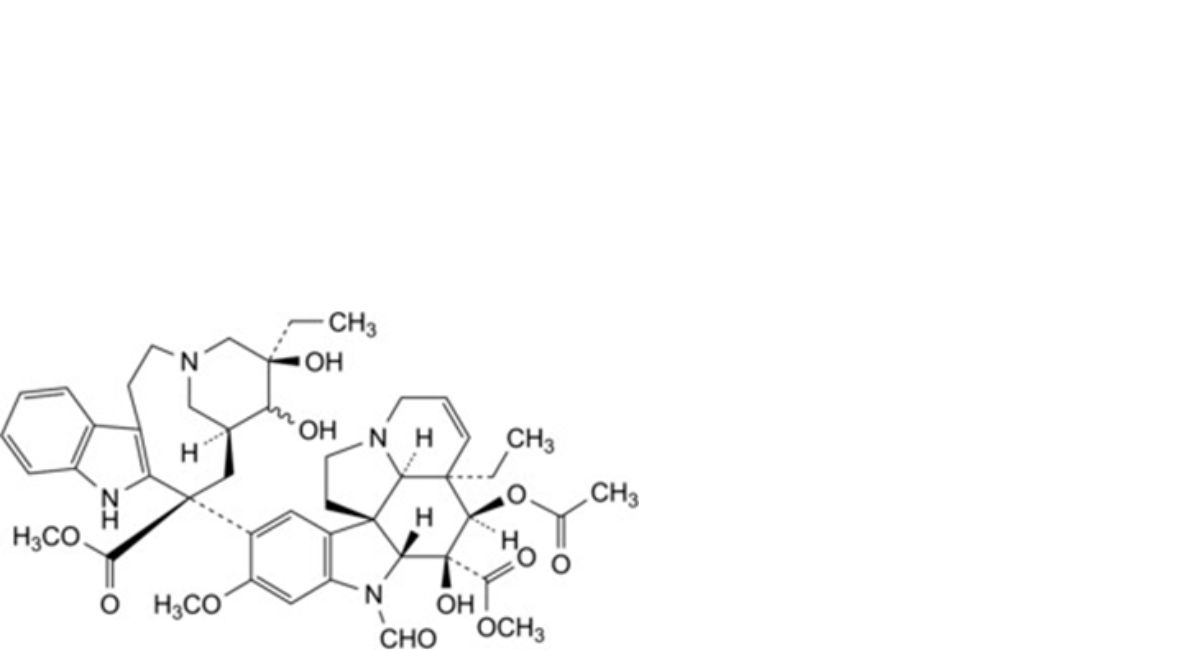
A. methyl (3aR,4R,5S,5aR,10bR,13aR)-4-(acetyloxy)-3a-ethyl-9-[(5R,7S,9S)-5-ethyl-5,6-dihydroxy-9- (methoxycarbonyl)-1,4,5,6,7,8,9,10-octahydro-2H-3,7-methanoazacycloundecino[5,4-b]indol-9-yl]-6-formyl-5-hydroxy-8- methoxy-3a,4,5,5a,6,11,12,13a-octahydro-1H-indolizino[8,1-cd]carbazole-5-carboxylate (3′-hydroxy-VCR),
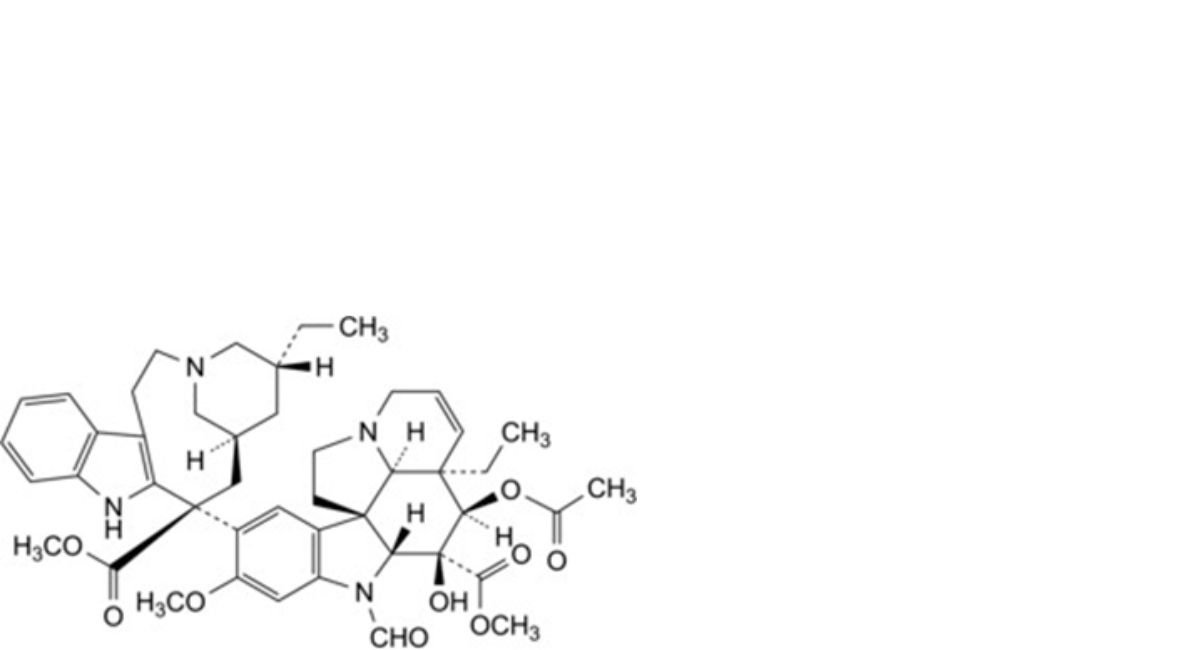
B. methyl (3aR,4R,5S,5aR,10bR,13aR)-4-(acetyloxy)-3a-ethyl-9-[(5R,7S,9S)-5-ethyl-9-(methoxycarbonyl)-1,4,5,6,7,8,9,10-octahydro-2H-3,7-methanoazacycloundecino[5,4-b]indol-9-yl]-6-formyl-5-hydroxy-8- methoxy-3a,4,5,5a,6,11,12,13a-octahydro-1H-indolizino[8,1-cd]carbazole-5-carboxylate (4′-deoxyvincristine),
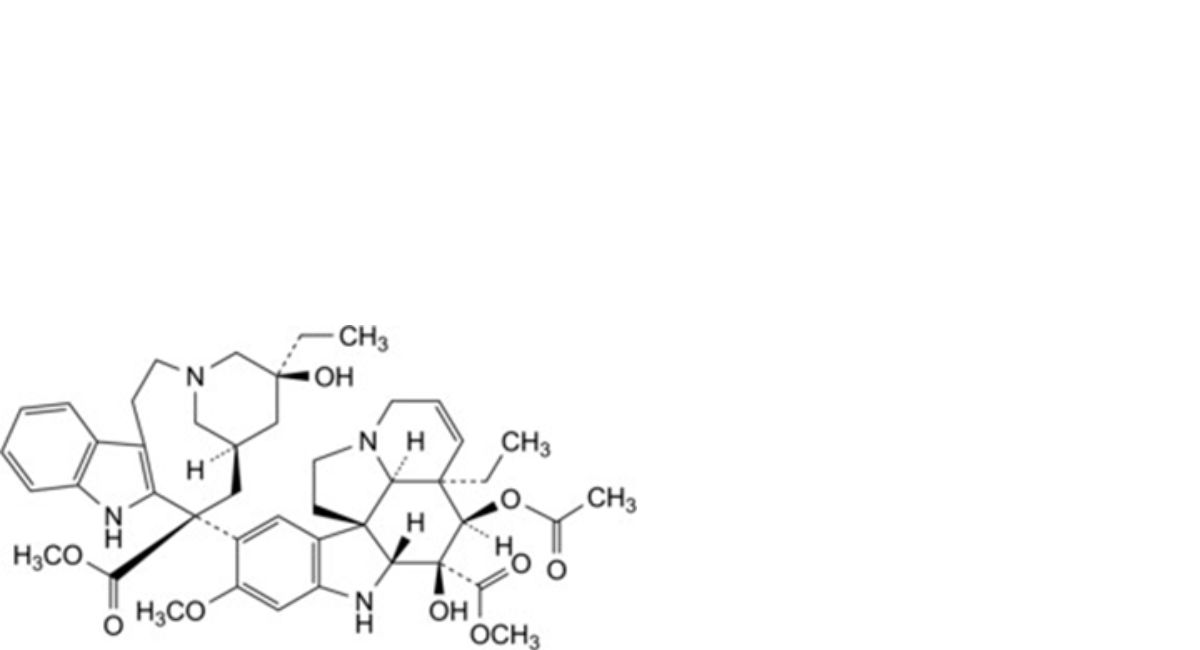
C. methyl (3aR,4R,5S,5aR,10bS,13aR)-4-(acetyloxy)-3a-ethyl-9-[(5S,7R,9S)-5-ethyl-5-hydroxy-9-(methoxycarbonyl)-1,4,5,6,7,8,9,10-octahydro-2H-3,7-methanoazacycloundecino[5,4-b]indol-9-yl]-5-hydroxy-8-methoxy- 3a,4,5,5a,6,11,12,13a-octahydro-1H-indolizino[8,1-cd]carbazole-5-carboxylate (N-desmethylvinblastine),
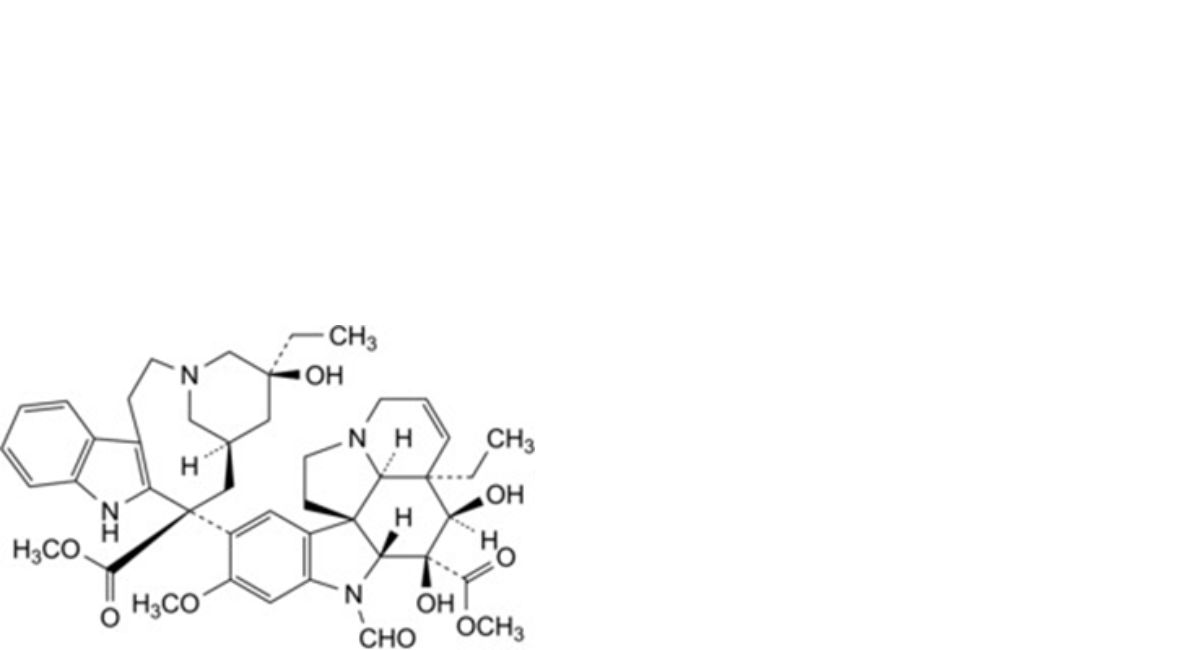
D. methyl (3aR,4R,5S,5aR,10bR,13aR)-3a-ethyl-9-[(5S,7R,9S)-5-ethyl-5-hydroxy-9-(methoxycarbonyl)-1,4,5,6,7,8,9,10- octahydro-2H-3,7-methanoazacycloundecino[5,4-b]indol-9-yl]-6-formyl-4,5-dihydroxy-8-methoxy-3a,4,5,5a,6,11,12,13a- octahydro-1H-indolizino[8,1-cd]carbazole-5-carboxylate (deacetylvincristine),
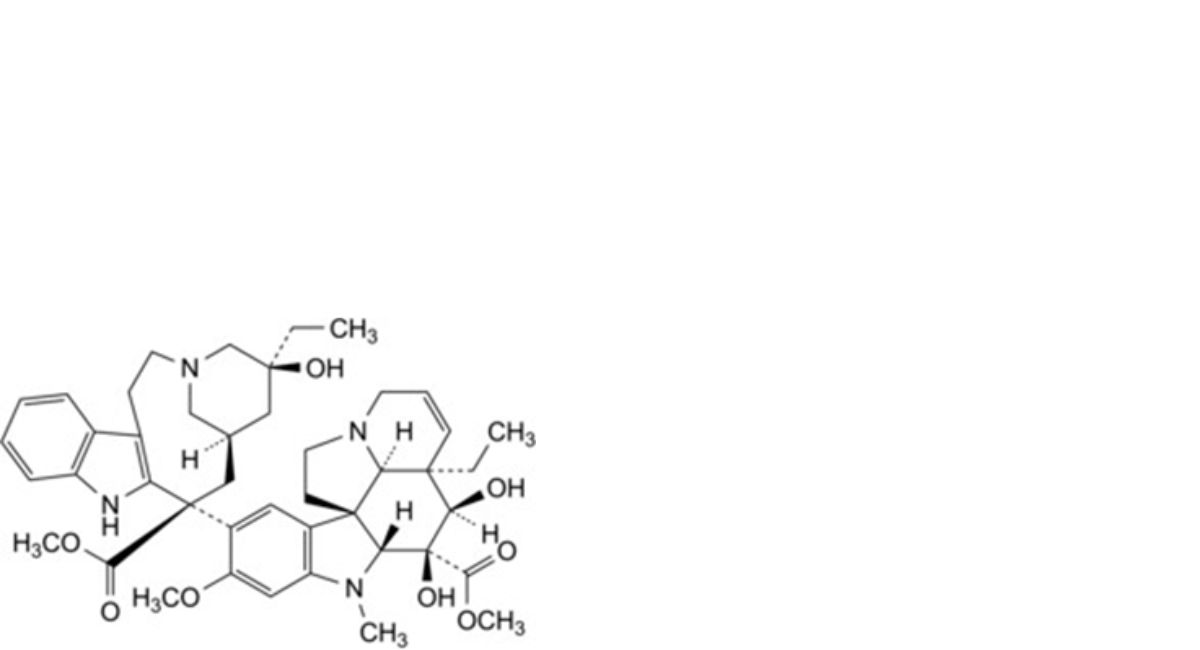
E. methyl (3aR,4R,5S,5aR,10bR,13aR)-3a-ethyl-9-[(5S,7R,9S)-5-ethyl-5-hydroxy-9-(methoxycarbonyl)-1,4,5,6,7,8,9,10- octahydro-2H-3,7-methanoazacycloundecino[5,4-b]indol-9-yl]-4,5-dihydroxy-8-methoxy-6-methyl-3a,4,5,5a,6,11,12,13a-octahydro-1H-indolizino[8,1-cd]carbazole-5-carboxylate (deacetylvinblastine),
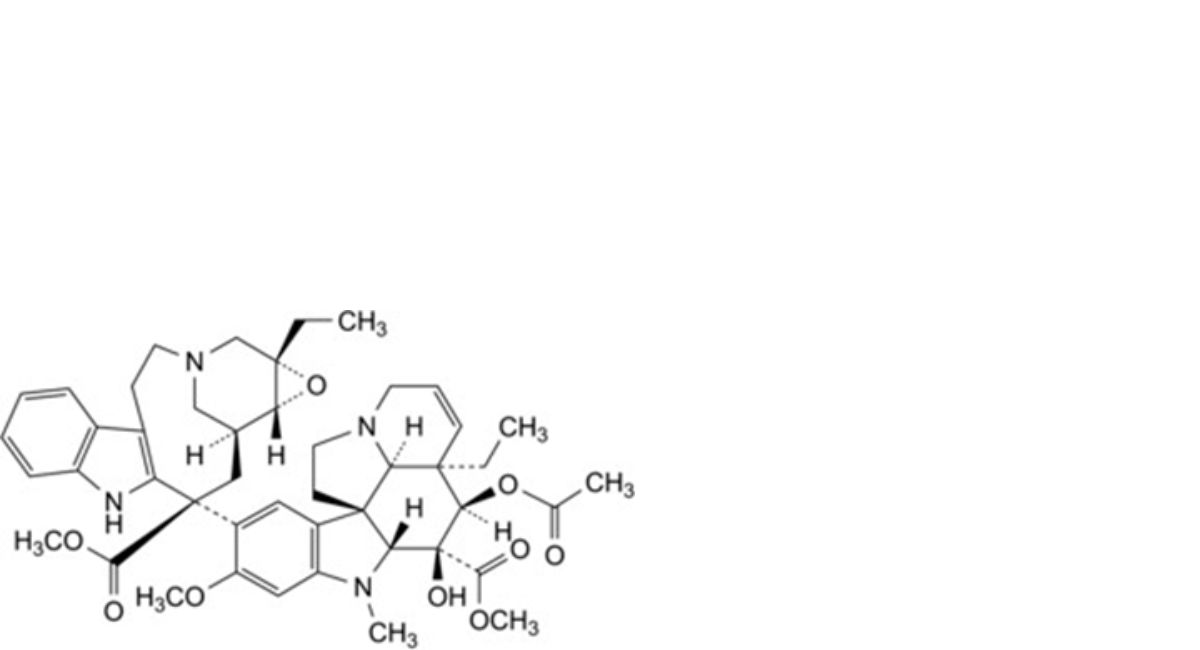
F. methyl (3aR,4R,5S,5aR,10bR,13aR)-4-(acetyloxy)-3a-ethyl-9-[(1aS,11S,13S,13aR)-1a-ethyl-11- (methoxycarbonyl)-1a,4,5,10,11,12,13,13a-octahydro-2H-3,13-methano-oxireno[9,10]azacycloundecino[5,4-b]indol-11- yl]-5-hydroxy-8-methoxy-6-methyl-3a,4,5,5a,6,11,12,13a-octahydro-1H-indolizino[8,1-cd]carbazole-5-carboxylate (leurosine),
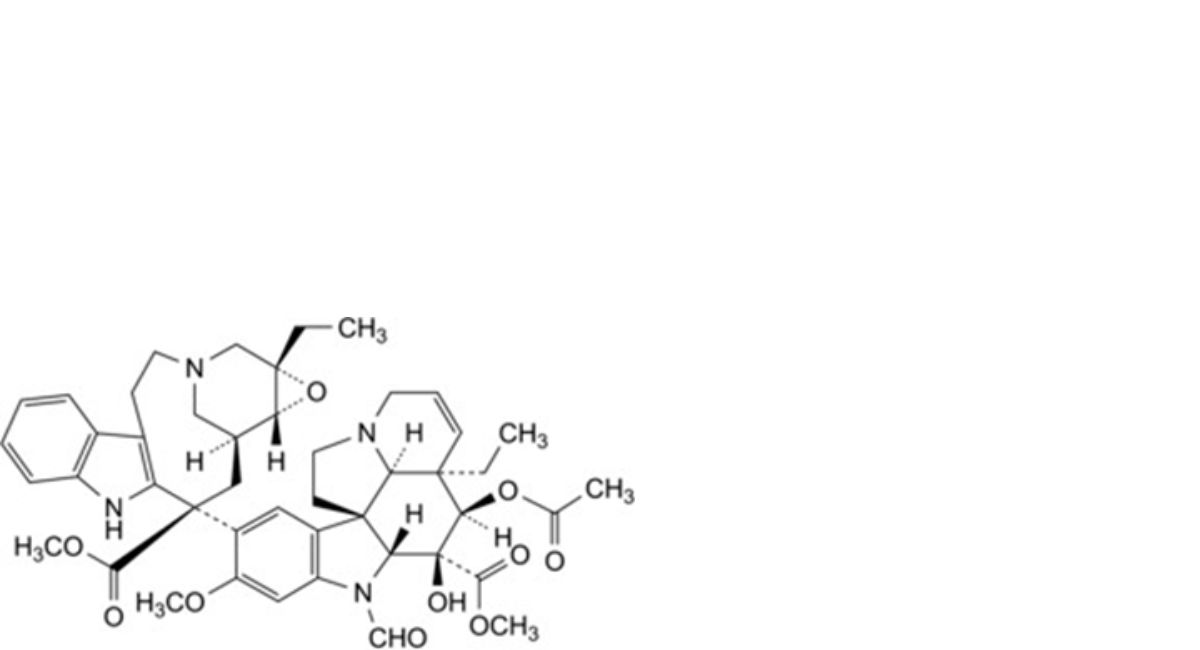
G. methyl (3aR,4R,5S,5aR,10bR,13aR)-4-(acetyloxy)-3a-ethyl-9-[(1aS,11S,13S,13aR)-1a-ethyl-11- (methoxycarbonyl)-1a,4,5,10,11,12,13,13a-octahydro-2H-3,13-methano-oxireno[9,10]azacycloundecino[5,4-b]indol-11- yl]-6-formyl-5-hydroxy-8-methoxy-3a,4,5,5a,6,11,12,13a-octahydro-1H-indolizino[8,1-cd]carbazole-5-carboxylate (formylleurosine),
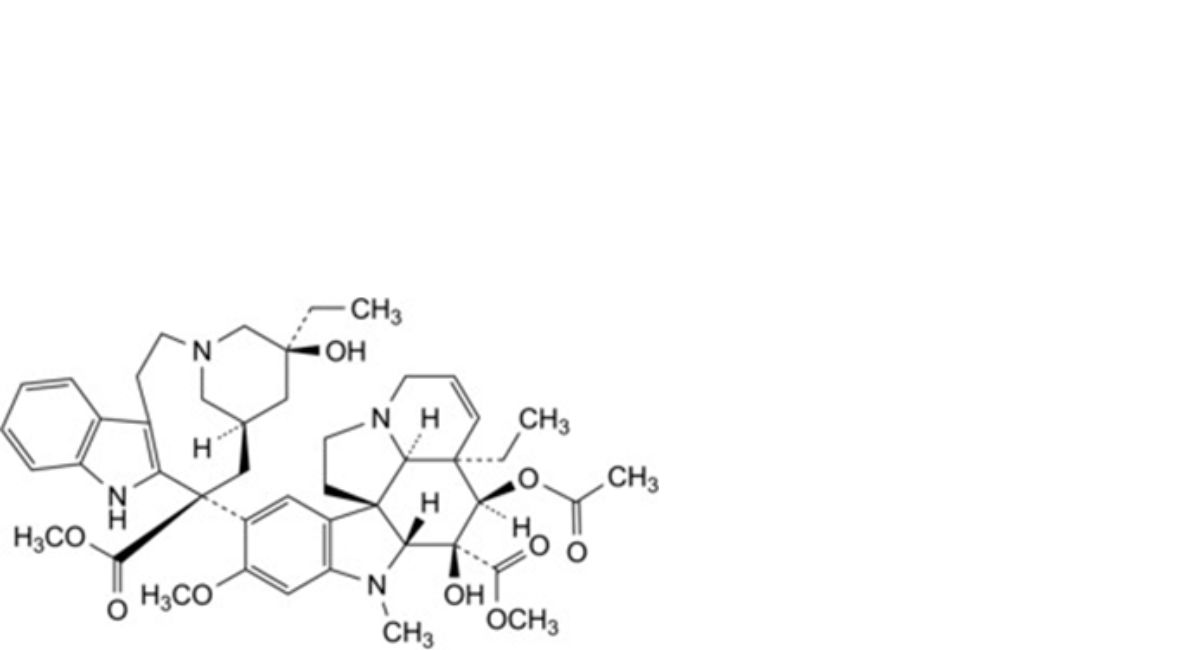
H. vinblastine.