Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Calcium channel blocker.
Preparations
Verapamil Prolonged-release Capsules
Verapamil Prolonged-release Tablets Verapamil Injection
Verapamil Oral Solution Verapamil Tablets
DEFINITION
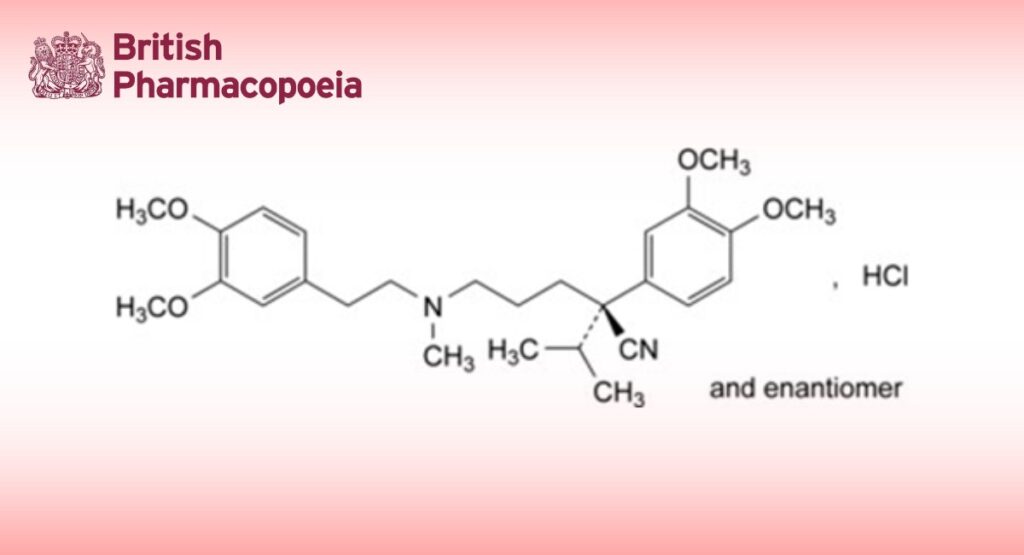
(2RS)-2-(3,4-Dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl](methyl)amino]-2-(propan-2-yl)pentanenitrile hydrochloride.
Content
99.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Soluble in water, freely soluble in methanol, sparingly soluble in ethanol (96 per cent).
IDENTIFICATION
First identification: B, D.
Second identification: A, C, D.
A. Ultraviolet and visible absorption spectrophotometry (2.2.25).
Test solution Dissolve 20.0 mg in a 1.03 g/L solution of hydrochloric acid R and dilute to 100.0 mL with the same acid. Dilute 5.0 mL of this solution to 50.0 mL with a 1.03 g/L solution of hydrochloric acid R.
Spectral range 210-340 nm.
Absorption maxima 229 nm and 278 nm.
Shoulder 282 nm.
Absorbance ratio A278/A229 = 0.35 to 0.39.
B. Infrared absorption spectrophotometry (2.2.24).
Comparison verapamil hydrochloride CRS.
C. Thin-layer chromatography (2.2.27).
Test solution Dissolve 10 mg of the substance to be examined in methylene chloride R and dilute to 5 mL with the same solvent.
Reference solution (a) Dissolve 20 mg of verapamil hydrochloride CRS in methylene chloride R and dilute to 10 mL with the same solvent.
Reference solution (b) Dissolve 5 mg of papaverine hydrochloride CRS in reference solution (a) and dilute to 5 mL with the same solution.
Plate TLC silica gel F254 plate R.
Mobile phase diethylamine R, cyclohexane R (15:85 V/V). Application 5 µL.
Development Over 3/4 of the plate.
Drying In air.
Detection Examine in ultraviolet light at 254 nm.
System suitability Reference solution (b):
— the chromatogram shows 2 clearly separated principal spots.
Results The principal spot in the chromatogram obtained with the test solution is similar in position and size to the principal spot in the chromatogram obtained with reference solution (a).
D. Dissolve about 28 mg in 2 mL of methanol R. The solution gives reaction (a) of chlorides (2.3.1); use methanol R instead of water R to wash the precipitate.
TESTS
Solution S
Dissolve 1.0 g in carbon dioxide-free water R with gentle heating and dilute to 20.0 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and colourless (2.2.2, Method II).
pH (2.2.3)
4.5 to 6.0 for solution S.
Optical rotation (2.2.7)
-0.10° to + 0.10°, determined on solution S.
Related substances
Liquid chromatography (2.2.29).
Solvent mixture Mobile phase B, mobile phase A (37:63 V/V).
Test solution Dissolve 25.0 mg of the substance to be examined in the solvent mixture and dilute to 10.0 mL with the solvent mixture.
Reference solution (a) Dissolve 5 mg of verapamil hydrochloride CRS, 5 mg of verapamil impurity I CRS and 5 mg of verapamil impurity M CRS in the solvent mixture and dilute to 20 mL with the solvent mixture. Dilute 1 mL of this solution to 10 mL with the solvent mixture.
Reference solution (b) Dilute 1.0 mL of the test solution to 100.0 mL with the solvent mixture. Dilute 1.0 mL of this solution to 10.0 mL with the solvent mixture.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: end-capped polar-embedded octadecylsilyl amorphous organosilica polymer R (5 µm).
Mobile phase:
— mobile phase A: 6.97 g/L solution of dipotassium hydrogen phosphate R adjusted to pH 7.20 with phosphoric acid R;
— mobile phase B: acetonitrile R;
| Time (min) | Mobile phase A (per cent V/V) | Mobile phase B (per cent V/V) |
| 0 – 22 | 63 | 37 |
| 22 – 27 | 63 → 35 | 37 → 65 |
| 27 – 45 | 35 | 65 |
Flow rate 1.5 mL/min.
Detection Spectrophotometer at 278 nm.
Injection 10 µL.
Relative retention With reference to verapamil (retention time = about 15 min): impurity I = about 1.3; impurity M = about 2.4.
System suitability Reference solution (a):
— resolution: minimum 4.5 between the peaks due to verapamil and impurity I;
— impurity M elutes from the column.
Limits:
— unspecified impurities: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (0.10 per cent);
— total: not more than 3 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.3 per cent);
— disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 per cent).
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.400 g in 50 mL of anhydrous ethanol R and add 5.0 mL of 0.01 M hydrochloric acid. Titrate with 0.1 M sodium hydroxide, determining the end-point potentiometrically (2.2.20). Measure the volume added between the 2 points of inflexion.
1 mL of 0.1 M sodium hydroxide is equivalent to 49.11 mg of C27H39ClN2O4.
STORAGE
Protected from light.
IMPURITIES
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) A, B, C, D, E, F, G, H, I, J, K, L, M, N, O, P.
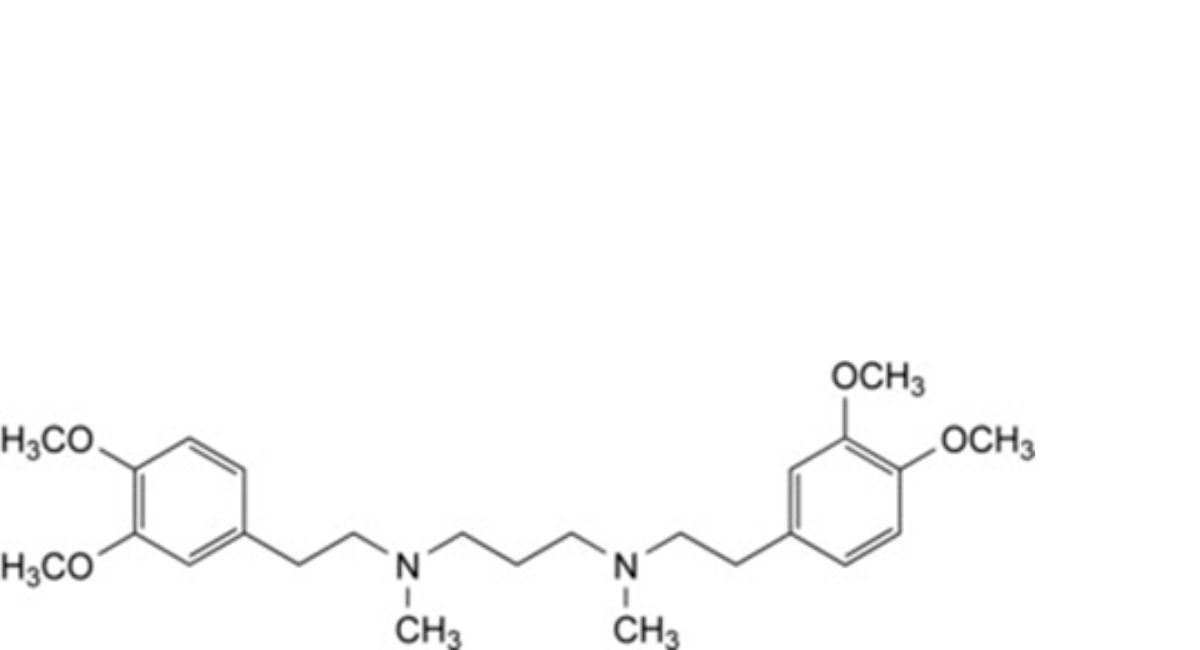
A. N1,N3-bis[2-(3,4-dimethoxyphenyl)ethyl]-N1,N3-dimethylpropane-1,3-diamine,
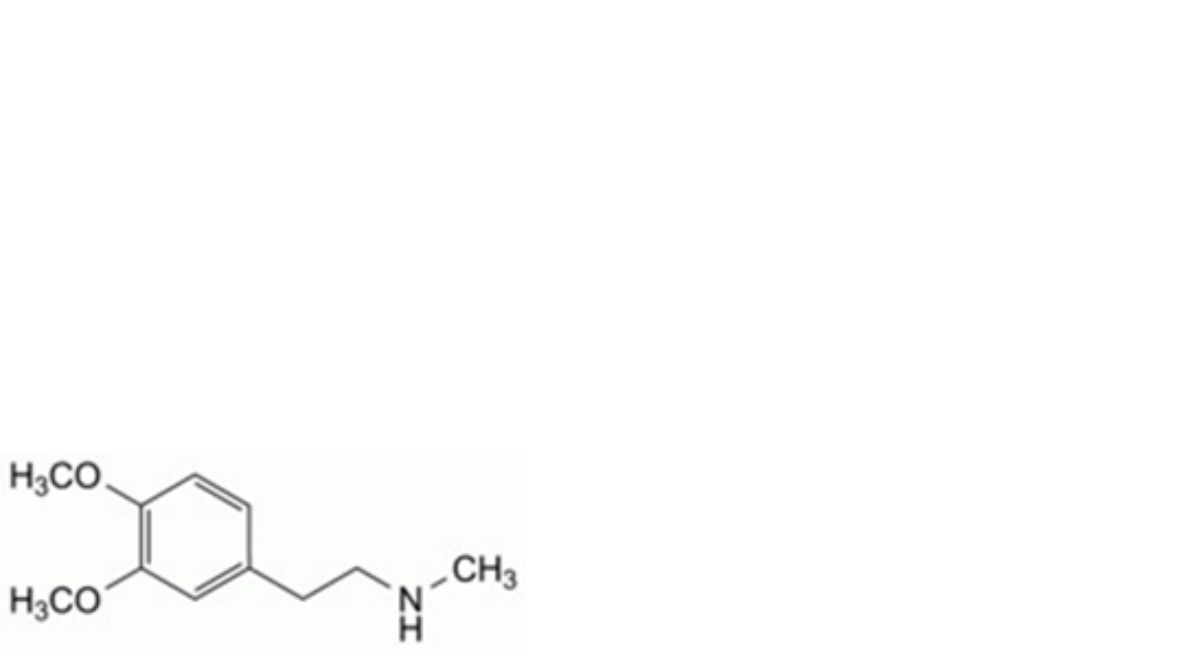
B. 2-(3,4-dimethoxyphenyl)-N-methylethan-1-amine,
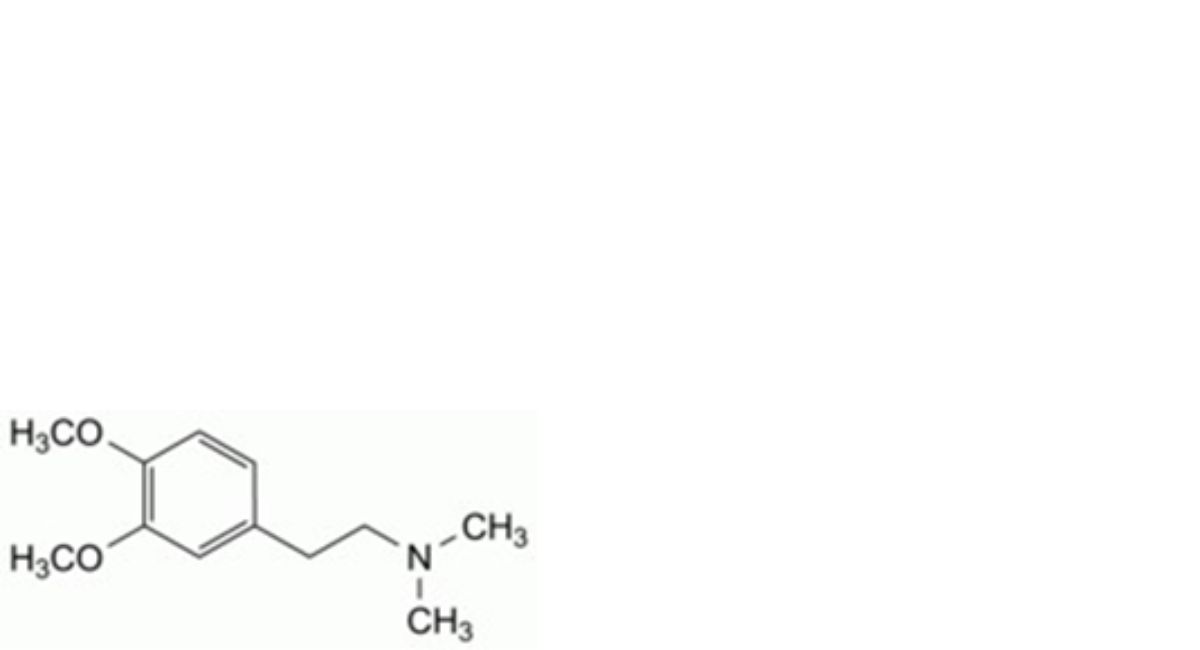
C. 2-(3,4-dimethoxyphenyl)-N,N-dimethylethan-1-amine,
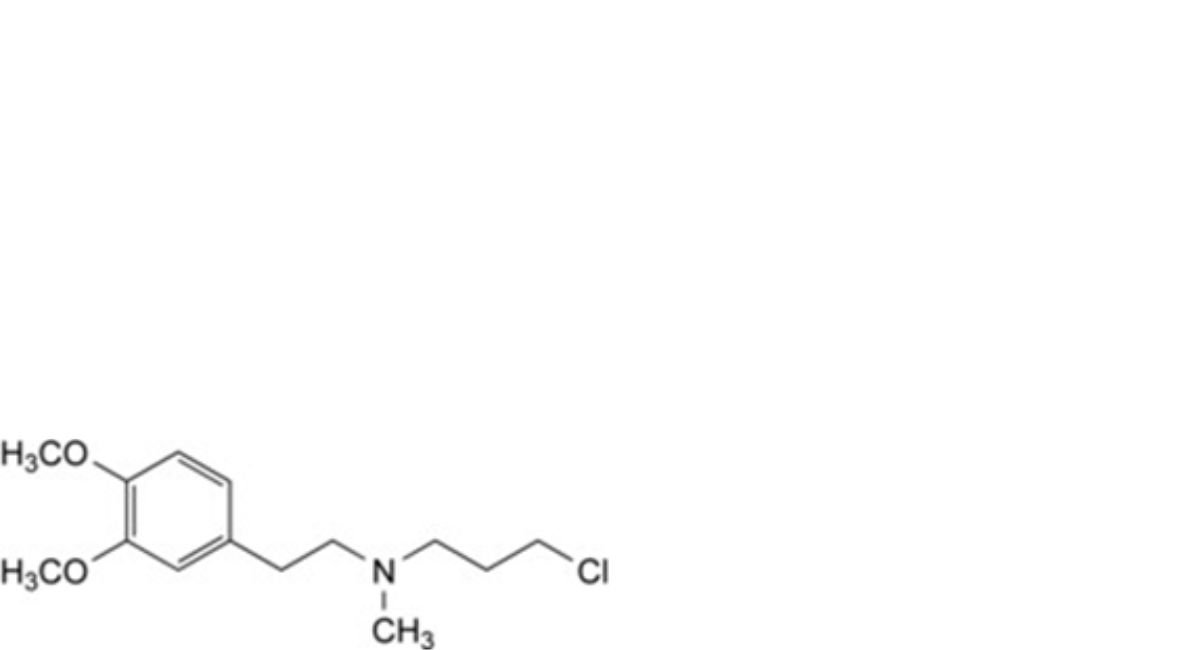
D. 3-chloro-N-[2-(3,4-dimethoxyphenyl)ethyl]-N-methylpropan-1-amine,
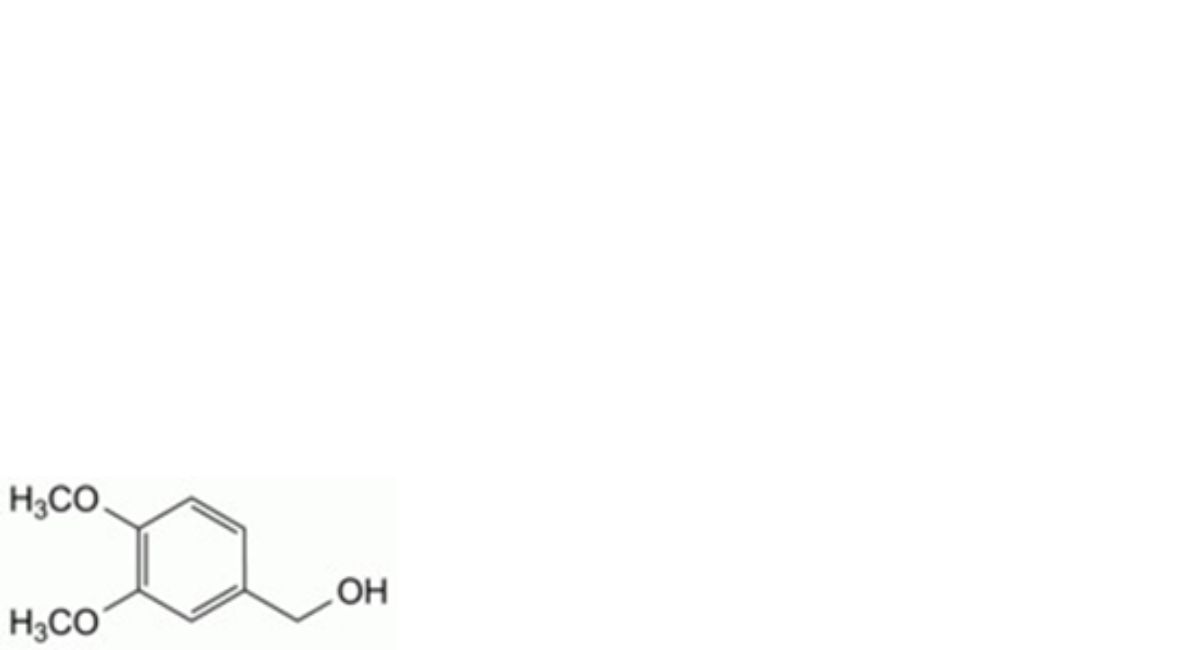
E. (3,4-dimethoxyphenyl)methanol,
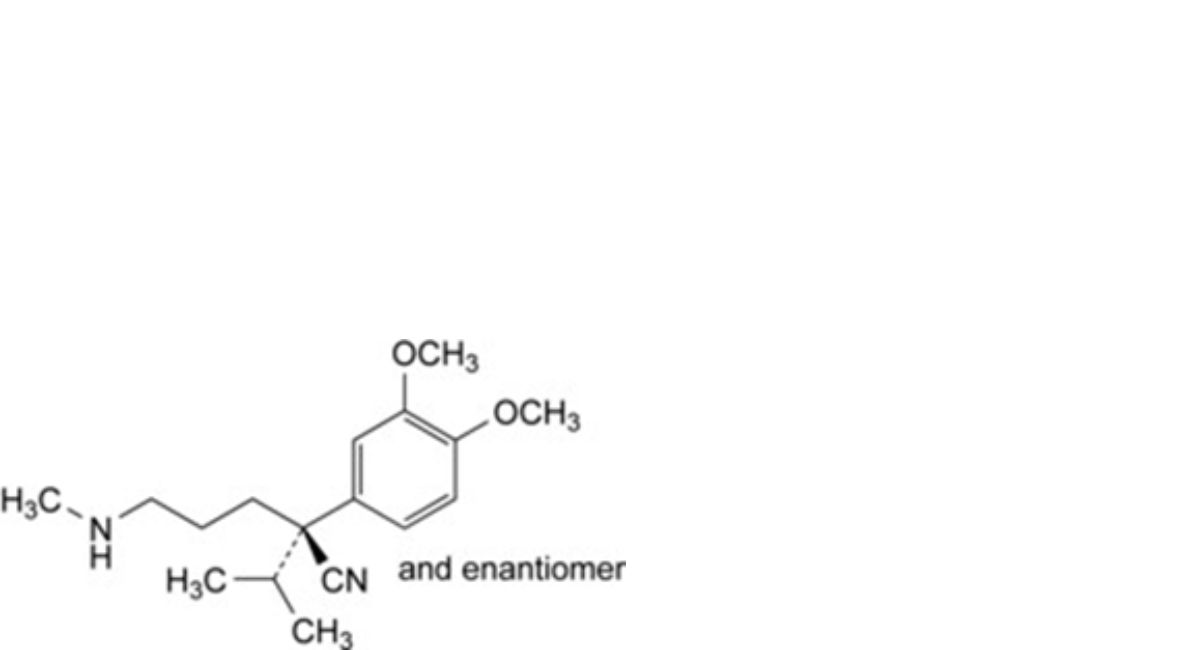
F. (2RS)-2-(3,4-dimethoxyphenyl)-5-(methylamino)-2-(propan-2-yl)pentanenitrile,

G. 3,4-dimethoxybenzaldehyde,
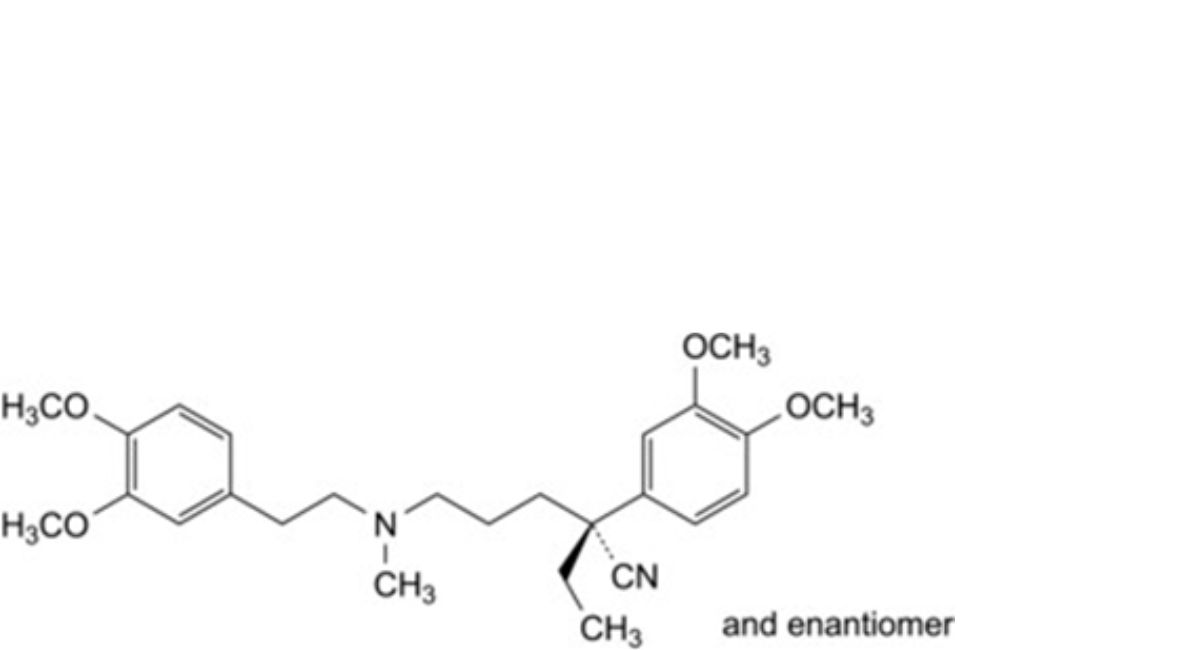
H. (2RS)-2-(3,4-dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl](methyl)amino]-2-ethylpentanenitrile,
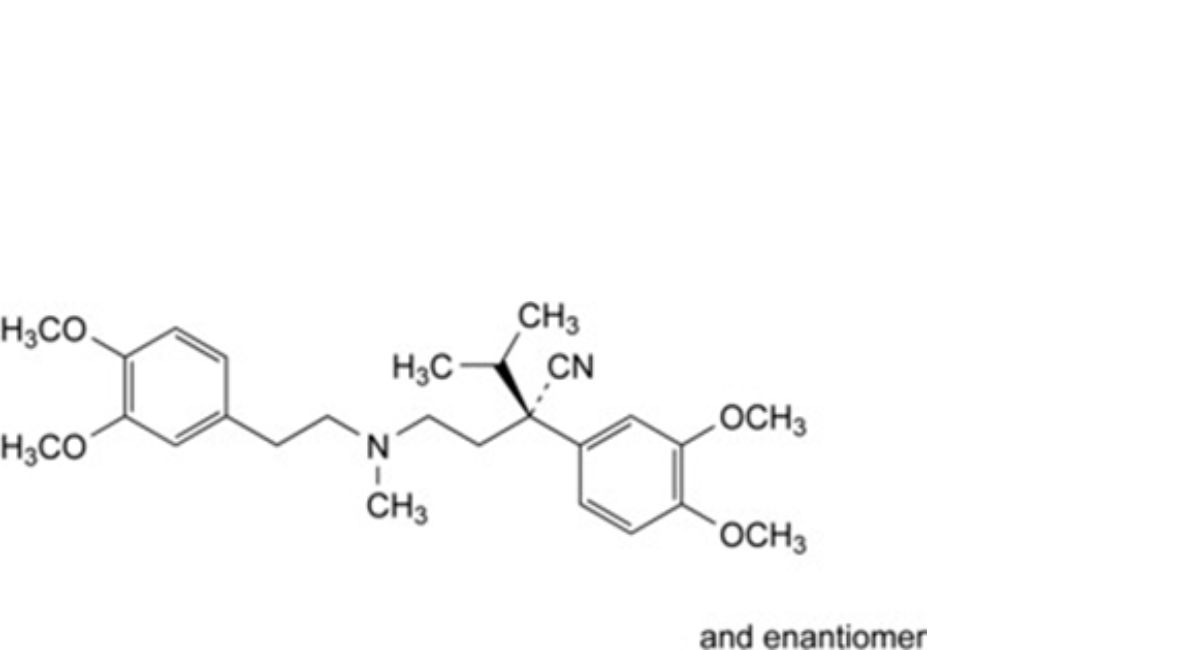
I. (2RS)-2-(3,4-dimethoxyphenyl)-4-[[2-(3,4-dimethoxyphenyl)ethyl](methyl)amino]-2-(propan-2-yl)butanenitrile,
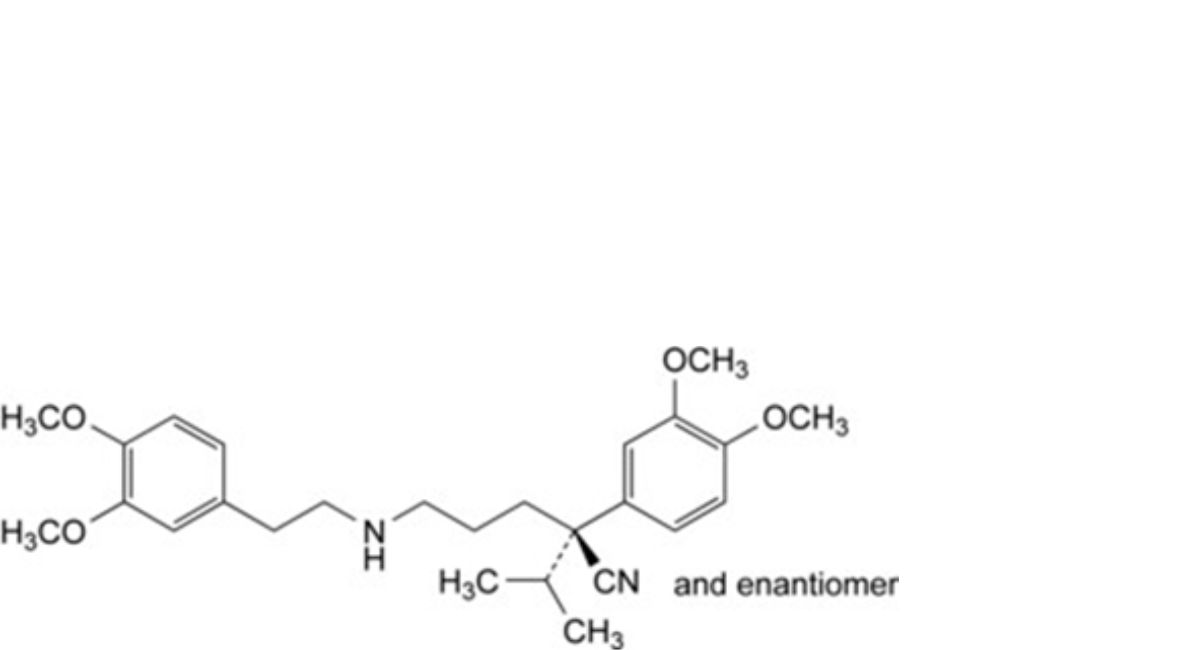
J. (2RS)-2-(3,4-dimethoxyphenyl)-5-[[2-(3,4-dimethoxy-phenyl)ethyl]amino]-2-(propan-2-yl)pentanenitrile (norverapamil),
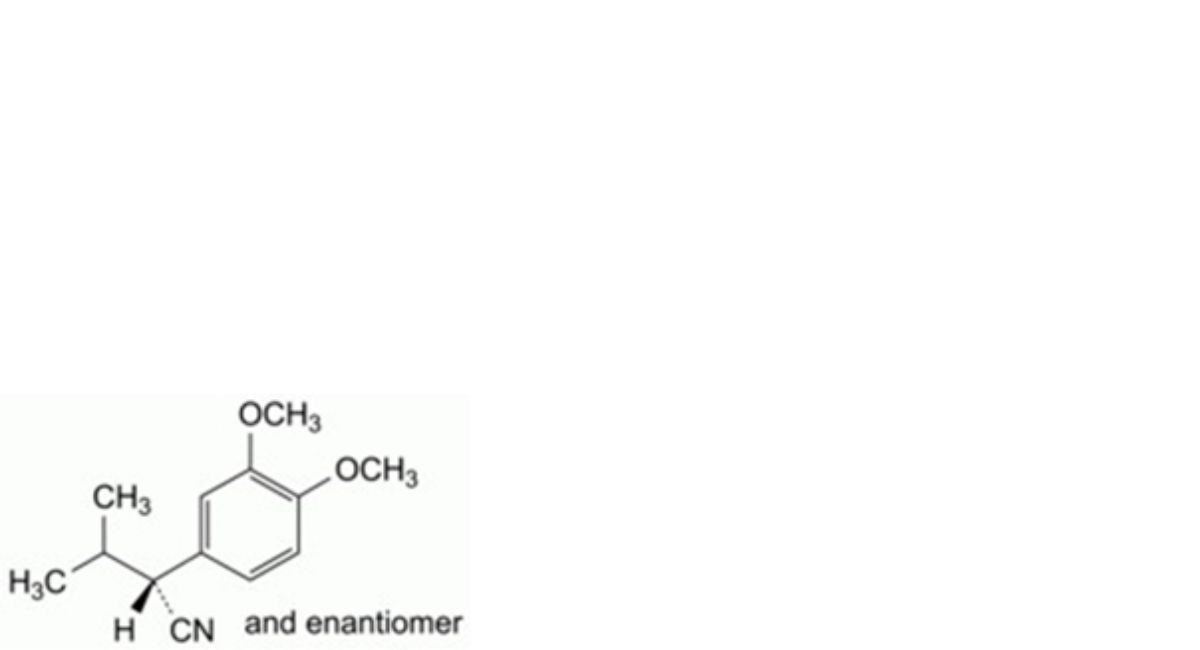
K. (2RS)-2-(3,4-dimethoxyphenyl)-3-methylbutanenitrile,
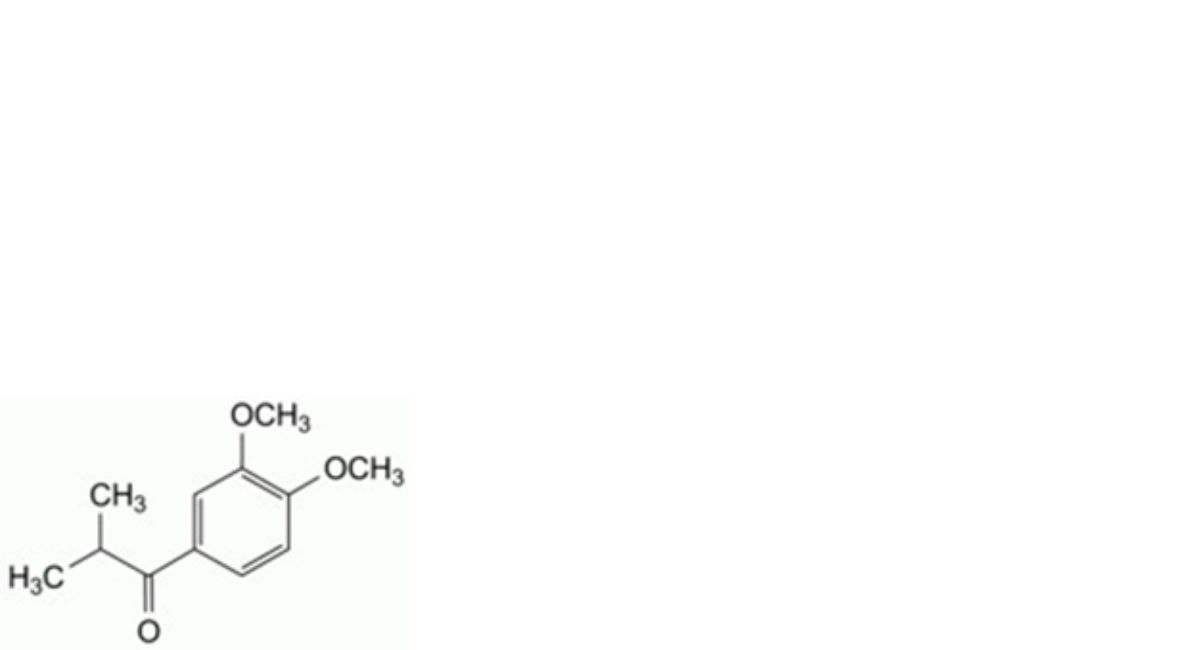
L. 1-(3,4-dimethoxyphenyl)-2-methylpropan-1-one,
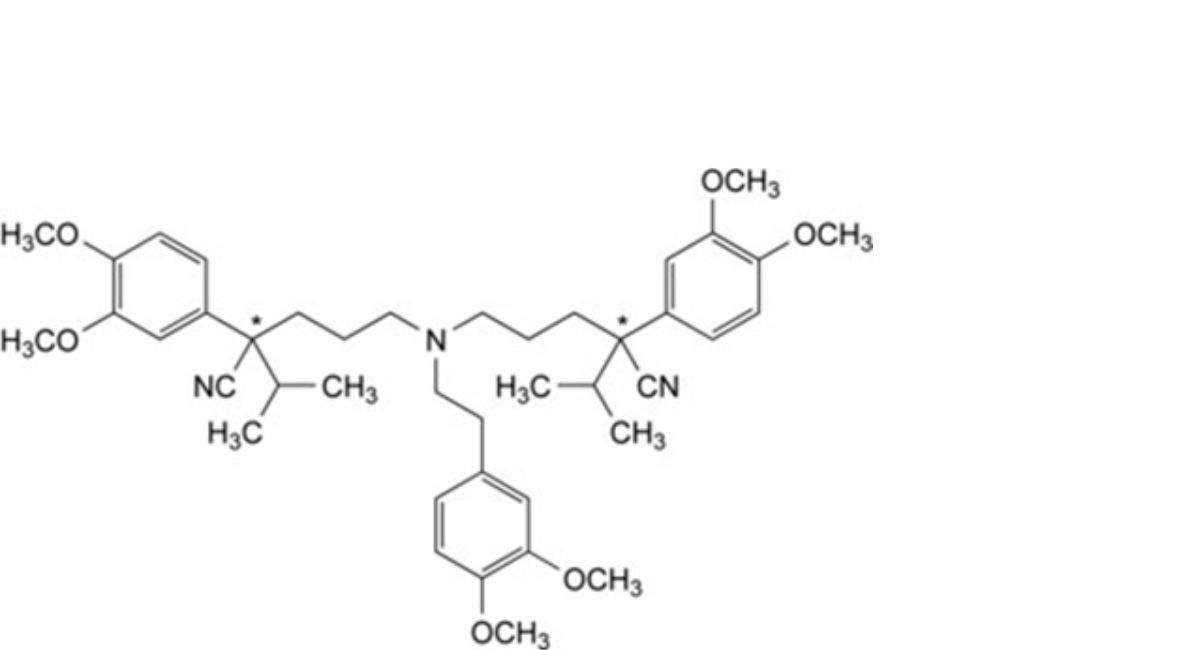
M. 5,5′-[[2-(3,4-dimethoxyphenyl)ethyl]azanediyl]bis[(2Ξ)-2-(3,4-dimethoxyphenyl)-2-(propan-2-yl)pentanenitrile],
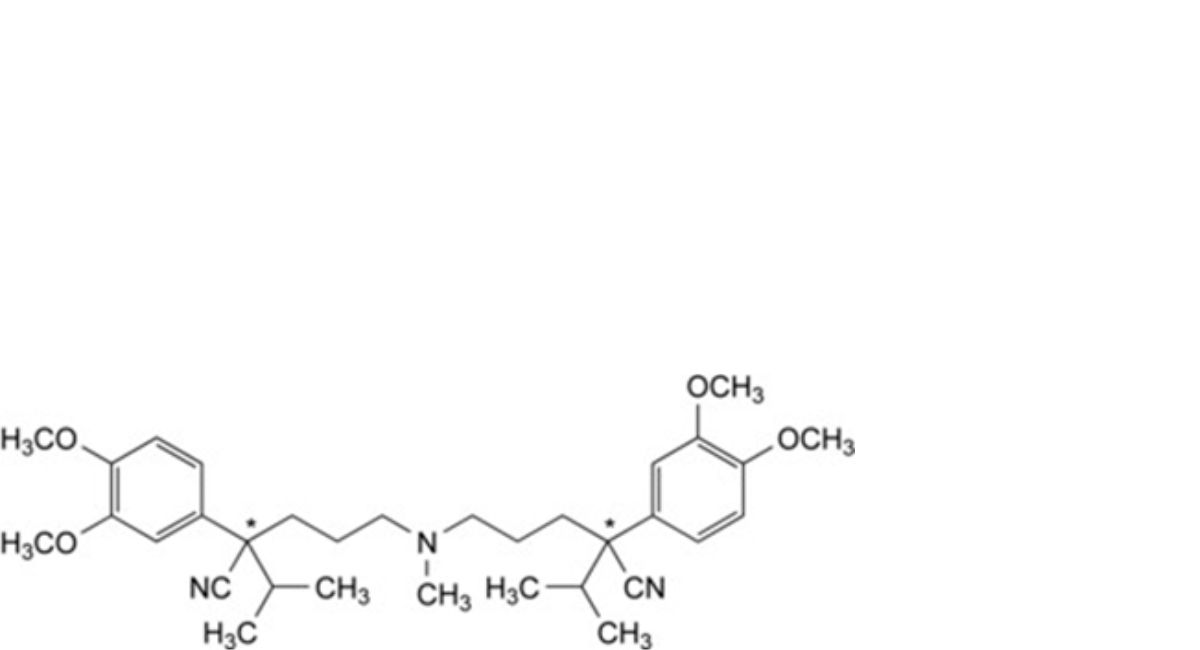
N. 5,5′-(methylazanediyl)bis[(2Ξ)-2-(3,4-dimethoxyphenyl)-2-(propan-2-yl)pentanenitrile],
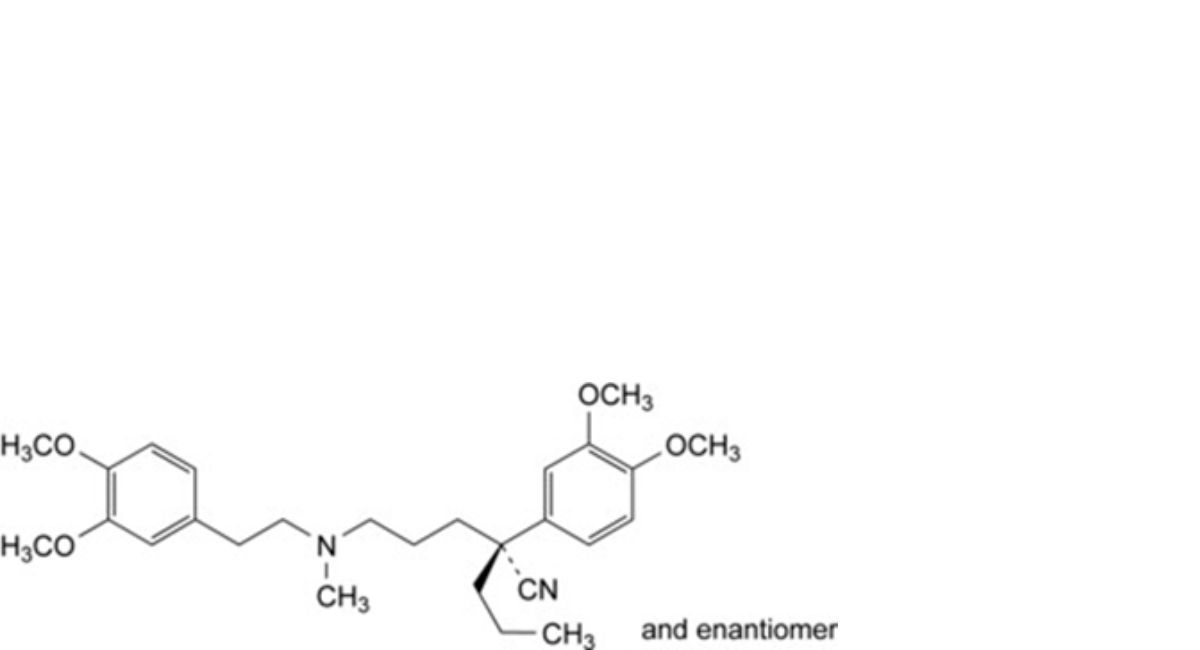
O. (2RS)-2-(3,4-dimethoxyphenyl)-5-[[2-(3,4-dimethoxy-phenyl)ethyl](methyl)amino]-2-propylpentanenitrile,
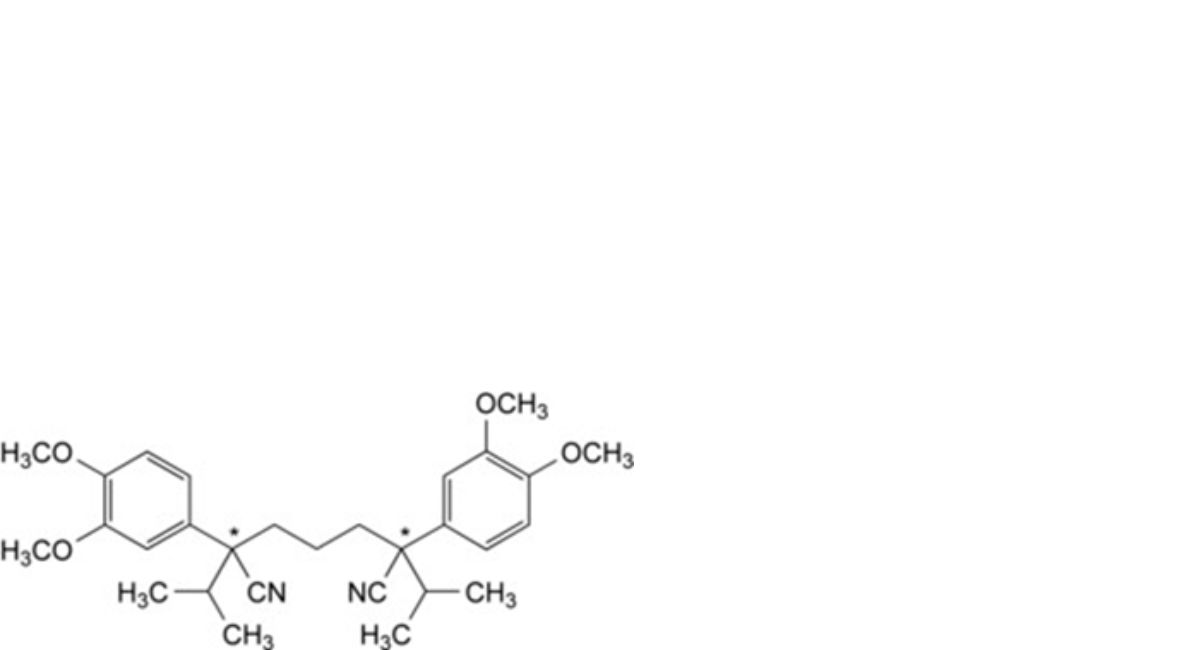
P. (2Ξ,6Ξ)-2,6-bis(3,4-dimethoxyphenyl)-2,6-di(propan-2-yl)heptane-1,7-dinitrile.