Action and use
Inhibition of 5HT and noradrenaline reuptake; antidepressant.
DEFINITION
Venlafaxine Tablets contain Venlafaxine Hydrochloride.
The tablets comply with the requirements stated under Tablets and with the following requirements.
Content of venlafaxine, C17H27NO2
95.0 to 105.0% of the stated amount.
IDENTIFICATION
Shake a quantity of the powdered tablets containing the equivalent of 0.35 g of venlafaxine with 100 mL of a mixture of 30 volumes of cyclohexane and 70 volumes of dichloromethane for 30 minutes, filter and evaporate the filtrate to dryness. Wash the residue with a mixture of 30 volumes of cyclohexane and 70 volumes of dichloromethane, filter and dry the residue. The infrared absorption spectrum of the residue, Appendix II A, is concordant with the reference spectrum of venlafaxine hydrochloride (RS 439).
TESTS
Dissolution
Comply with the dissolution test for tablets and capsules, Appendix XII B1.
TEST CONDITIONS
(a) Use Apparatus 2, rotating the paddle at 50 revolutions per minute.
(b) Use 900 mL of water, at a temperature of 37°, as the medium.
PROCEDURE
(1) After 45 minutes withdraw a sample of the medium and measure the absorbance of the filtered sample, suitably diluted with the dissolution medium if necessary, to give a solution expected to contain the equivalent of about 0.0025% w/v of venlafaxine, at the maximum at 274 nm, Appendix II B using water in the reference cell.
(2) Measure the absorbance of a 0.0025% w/v solution of venlafaxine hydrochloride BPCRS using water in the reference cell.
DETERMINATION OF CONTENT
Calculate the total content of venlafaxine, C17H27NO2, in the medium from the absorbances obtained and using the declared content of C17H27NO2,HCl in venlafaxine hydrochloride BPCRS. Each mg of C17H27NO2,HCl is equivalent to 0.884 mg of C17H27NO2.
LIMITS
The amount of venlafaxine released is not less than 75% (Q) of the stated amount.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions in the mobile phase A.
(1) Mix with the aid of ultrasound a quantity of the powdered tablets containing the equivalent of 200 mg of venlafaxine with 80 mL of a 2.4% v/v solution of orthophosphoric acid, shake for a further 30 minutes, cool, add sufficient water to produce 100 mL, mix and centrifuge; use the supernatant liquid.
(2) Dilute 1 volume of solution (1) to 500 volumes.
(3) 0.2% w/v of venlafaxine impurity standard BPCRS.
(4) Dilute 25 volumes of solution (2) to 100 volumes.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (25 cm × 4.6 mm) packed with octadecylsilyl silica gel for chromatography (5 μm) (Partisil ODS 3 is suitable).
(b) Use gradient elution and the mobile phase described below.
(c) Use a flow rate of 1 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 226 nm.
(f) Inject 20 μL of each solution.
MOBILE PHASE
Mobile phase A: 1 volume of triethylamine, 20 volumes of acetonitrile and 80 volumes of water adjusted to pH 3.5 with orthophosphoric acid.
Mobile phase B: 1 volume of triethylamine, 50 volumes of acetonitrile and 50 volumes of water adjusted to pH 3.5 with orthophosphoric acid.
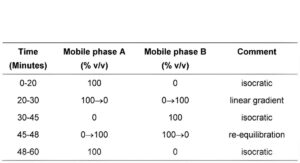
Under the prescribed conditions, the retention time of venlafaxine is about 13 minutes.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between the peaks due to impurity D and venlafaxine is at least 1.5.
LIMITS
In the chromatogram obtained with solution (1):
the area of any peak corresponding to impurity D or impurity F is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.2% of each);
the area of any other secondary peak is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.2%);
the sum of the areas of all the secondary peaks is not greater than 2.5 times the area of the principal peak in the chromatogram obtained with solution (2) (0.5%).
Disregard any peak with an area less than that of the principal peak in the chromatogram obtained with solution (4) (0.05%).
ASSAY
Weigh and powder 20 tablets. Carry out the method for liquid chromatography, Appendix III D, using the following solutions
in the mobile phase.
(1) Mix a quantity of the powdered tablets containing the equivalent of 50 mg of venlafaxine with 200 mL of a 0.2% v/v solution of orthophosphoric acid for 15 minutes with the aid of ultrasound and shake vigorously. Mix for a further 15 minutes with the aid of ultrasound, cool, add sufficient of a 0.2% v/v solution of orthophosphoric acid to produce 250 mL, mix and centrifuge. To 2 volumes of the supernatant liquid add sufficient of the mobile phase to produce 5 volumes.
(2) 0.009% w/v of venlafaxine hydrochloride BPCRS.
(3) 0.01% w/v of venlafaxine impurity standard BPCRS.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (15 cm × 4.6 mm) packed with octylsilyl silica gel for chromatography (5 μm) (Zorbax C8 is suitable)
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1 mL per minute.\
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 226 nm.
(f) Inject 20 μL of each solution.
MOBILE PHASE
25 volumes of acetonitrile and 75 volumes of a 1% v/v solution of triethylamine, previously adjusted to pH 3.0 with orthophosphoric acid.
Under the prescribed conditions, the retention time of venlafaxine is about 5 minutes, if necessary adjust the acetonitrile content of the mobile phase.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution factor between the peaks due to impurity D and venlafaxine is at least 1.0.
DETERMINATION OF CONTENT
Calculate the content of C17H27NO2 in the tablets using the declared content of C17H27NO2,HCl in venlafaxine hydrochloride BPCRS. Each mg of C17H27NO2,HCl is equivalent to 0.884 mg of C17H27NO2.
LABELLING
The quantity of active ingredient is stated in terms of the equivalent amount of venlafaxine.
IMPURITIES
The impurities limited by the requirements of this monograph include:
D. 1-[(1RS)-1-(4-methoxyphenyl)-2-(methylamino)ethyl]cyclohexanol (European Pharmacopoeia impurity D);
F. (2RS)-2-(cyclohex-1-enyl)-2-(4-methoxyphenyl)-N,N-dimethylethanamine (European Pharmacopoeia impurity F).






