NOTE: This monograph has been developed to cover unlicensed formulations.
Action and use
Glycopeptide antibacterial.
DEFINITION
Vancomycin Eye Drops are a sterile solution of Vancomycin Hydrochloride in Purified Water.
The eye drops comply with the requirements stated under Eye Preparations and with the following requirements. Where appropriate, the eye drops also comply with the requirements stated under Unlicensed Medicines.
IDENTIFICATION
A. In the test for Vancomycin B, the retention time of the principal peak in the chromatogram obtained with solution (1) is similar to that of the principal peak in the chromatogram obtained with solution (4).
B. Yield reaction A characteristic of chlorides, Appendix VI.
TESTS
Acidity
pH, 2.5 to 4.5, Appendix V L.
Vancomycin B
Carry out the method for liquid chromatography, Appendix III D, using the following solutions. Use the solutions within 4 hours of preparation.
(1) Dilute a volume of the eye drops with sufficient mobile phase A to produce a solution containing about 0.2% w/v of Vancomycin Hydrochloride.
(2) Dilute 1 volume of solution (1) to 25 volumes with mobile phase A.
(3) Dilute 1 volume of solution (2) to 40 volumes with mobile phase A.
(4) Heat a 0.050% w/v solution of vancomycin hydrochloride EPCRS in mobile phase A at 65° for 24 hours and allow to cool.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (25 cm × 4.6 mm) packed with end-capped octadecylsilyl silica gel for chromatography (5 μm) (Hypersil ODS is suitable).
(b) Use gradient elution and the mobile phase described below.
(c) Use a flow rate of 1 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 280 nm.
(f) Inject 20 μL of each solution.
MOBILE PHASE
Mobile phase A: To 4 volumes of triethylamine add 1996 volumes of water and adjust to pH 3.2 with orthophosphoric acid (solution A). Add 10 volumes of tetrahydrofuran and 70 volumes of acetonitrile to 920 volumes of solution A.
Mobile phase B: Add 10 volumes of tetrahydrofuran and 290 volumes of acetonitrile to 700 volumes of solution A. Use the following gradient.
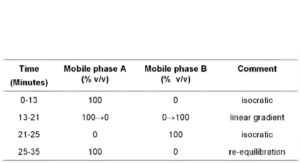
SYSTEM SUITABILITY
The test is not valid unless:
in the chromatogram obtained with solution (3), the principal peak has a signal-to-noise ratio of at least 5;
in the chromatogram obtained with solution (2), the symmetry factor of the vancomycin peak is not greater than 1.6;
in the chromatogram obtained with solution (4), the resolution between the two principal peaks is at least 5.0.
LIMITS
Not less than 88.0%, calculated using the following expression:

where,
Ab = area of the peak corresponding to vancomycin B in the chromatogram obtained with solution (2);
At = sum of the areas of the peaks corresponding to impurities in the chromatogram obtained with solution (1).
Related substances
Carry out the method for liquid chromatography, Appendix III D, using solutions (1), (2) and (3) described under Vancomycin B.
CHROMATOGRAPHIC CONDITIONS
The chromatographic conditions described under Vancomycin B may be used.
LIMITS
In the chromatogram obtained with solution (1) calculate the percentage content of each impurity using the following expression:

where,
Ai = area of an impurity peak in the chromatogram obtained with solution (1),
Ab = area of the peak corresponding to vancomycin B in the chromatogram obtained with solution (2),
At = sum of the areas of the peaks corresponding to impurities in the chromatogram obtained with solution (1).
The content of any impurity is not greater than 4.0% and the sum of the contents of any such impurities is not greater than 12.0%.
Disregard any peak with an area less than that of the principal peak in the chromatogram obtained with solution (3) (0.1%).
ASSAY
Carry out the microbiological assay of antibiotics, Appendix XIV A. The precision of the assay is such that the fiducial limits of error are not less than 95% and not more than 105% of the estimated potency.
The upper fiducial limit of error is not less than 95.0% and the lower fiducial limit of error is not more than 115.0% of the stated content.






