Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Keratolytic.
Preparation
Urea Cream
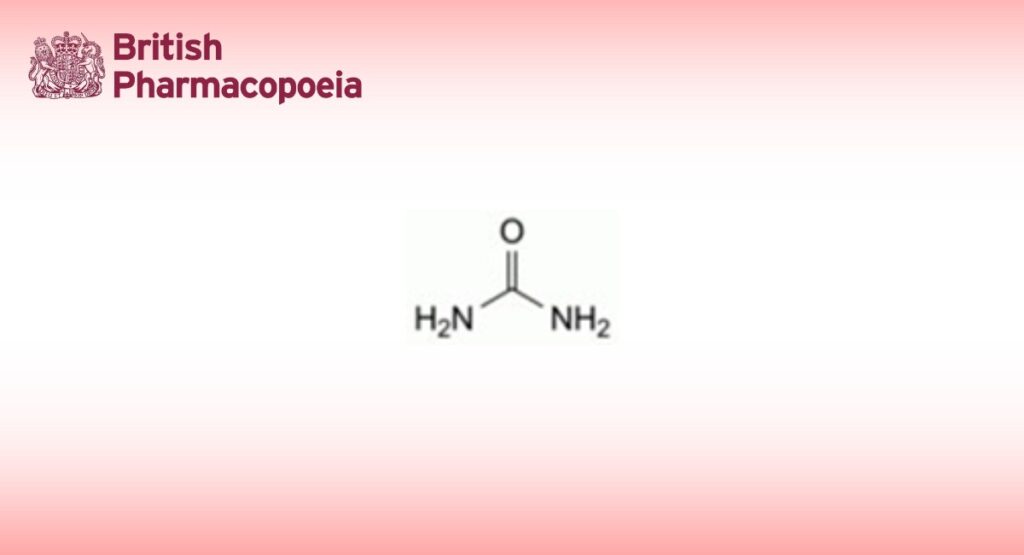
DEFINITION
Carbamide.
Content
98.5 per cent to 101.5 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder or transparent crystals, slightly hygroscopic.
Solubility
Very soluble in water, soluble in ethanol (96 per cent), practically insoluble in methylene chloride.
IDENTIFICATION
First identification: A, B.
Second identification: A, C, D.
A. Melting point (2.2.14): 132 °C to 135 °C.
B. Infrared absorption spectrophotometry (2.2.24).
Comparison urea CRS.
C. Dissolve 0.1 g in 1 mL of water R. Add 1 mL of nitric acid R. A white, crystalline precipitate is formed.
D. Heat 0.5 g in a test tube until it liquefies and the liquid becomes turbid. Cool, dissolve in a mixture of 1 mL of dilute sodium hydroxide solution R and 10 mL of water R and add 0.05 mL of copper sulfate solution R. A reddish-violet colour is produced.
TESTS
Solution S
Dissolve 10.0 g in water R and dilute to 50 mL with the same solvent.
Appearance of solution
The solution is clear (2.2.1) and colourless (2.2.2, Method II). To 2.5 mL of solution S add 7.5 mL of water R.
Alkalinity
To 2.5 mL of solution S add 7.5 mL of water R, 0.1 mL of methyl red solution R and 0.4 mL of 0.01 M hydrochloric acid. The solution is red to orange.
Biuret
Maximum 0.1 per cent.
To 10 mL of solution S add 5 mL of water R, 0.5 mL of a 5 g/L solution of copper sulfate pentahydrate R and 0.5 mL of strong sodium hydroxide solution R. Allow to stand for 5 min. Any reddish-violet colour in the solution is not more intense than that in a standard prepared at the same time in the same manner using 10 mL of a 0.2 g/L solution of biuret R.
Ammonium (2.4.1)
Maximum 500 ppm, determined on 0.1 mL of solution S.
Loss on drying (2.2.32)
Maximum 1.0 per cent, determined on 1.000 g by drying in an oven at 105 °C for 1 h.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.2000 g in water R and dilute to 50.0 mL with the same solvent. Introduce 1.0 mL of the solution into a combustion flask. Add 4 g of a powdered mixture of 100 g of dipotassium sulfate R, 5 g of copper sulfate pentahydrate R and 2.5 g of selenium R, and 3 glass beads. Wash any adhering particles from the neck into the flask with 5 mL of sulfuric acid R, allowing it to run down the sides of the flask, and mix the contents by rotation. Close the mouth of the flask loosely, for example by means of a glass bulb with a short stem, to avoid excessive loss of sulfuric acid. Heat gradually at first, then increase the temperature until there is vigorous boiling with condensation of sulfuric acid in the neck of the flask; take precautions to prevent the upper part of the flask from becoming overheated. Continue the heating for 30 min. Cool, dissolve the solid material by cautiously adding to the mixture 25 mL of water R, cool again and place in a steam-distillation apparatus. Add 30 mL of strong sodium hydroxide solution R and distil immediately by passing steam through the mixture. Collect the distillate in 15 mL of a 40 g/L solution of boric acid R to which has been added 0.2 mL of methyl red mixed solution R and enough water R to cover the tip of the condenser. Towards the end of the distillation, lower the receiver so that the tip of the condenser is above the surface of the acid. Take precautions to prevent any water on the outer surface of the condenser from reaching the contents of the receiver. Titrate the distillate with 0.01 M sulfuric acid.
1 mL of 0.01 M sulfuric acid is equivalent to 0.6006 mg of CH4N2O.
STORAGE
In an airtight container.