(Tylosin Phosphate Bulk Solution for Veterinary Use, Ph. Eur. monograph 1661)
NOTE: The name Tylosin Phosphate was formerly used in the United Kingdom.
Action and use
Macrolide antibacterial.
DEFINITION
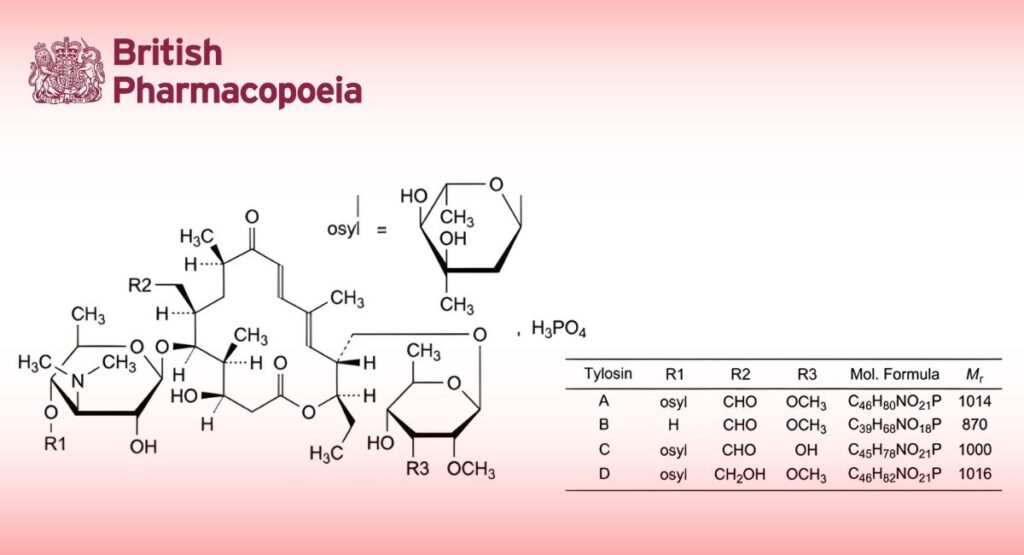
Solution of the dihydrogen phosphate of a mixture of macrolide antibiotics produced by certain strains of Streptomyces fradiae. The main component is 2-[(4R,5S,6S,7R,9R,11E,13E,15R,16R)-15-[[(6-deoxy-2,3-di-O-methyl-β-D-allopyranosyl)oxy]methyl]-6-[[3,6-dideoxy-4-O-(2,6-dideoxy-3-C-methyl-α-L-ribo-hexopyranosyl)-3-(dimethylamino)-β-D-glucopyranosyl]oxy]-16-ethyl-4-hydroxy-5,9,13-trimethyl-2,10-dioxo-1-oxacyclohexadeca-11,13-dien-7-yl]acetaldehyde monophosphate (tylosin monophosphate, tylosin A monophosphate). The monophosphates of tylosin B (desmycosin, 4′-O-demycarosyltylosin), tylosin C (macrocin, 3′′′-O-demethyltylosin) and tylosin D (relomycin, 19-deoxo-19-hydroxytylosin) may also be present. The solution also contains sodium dihydrogen phosphate.
Content
Minimum 815 IU/mg of dry residue. Tylosins A, B, C and D contribute to the potency.
CHARACTERS
Appearance
Yellow or brownish-yellow, viscous liquid.
Solubility
Miscible with water.
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24).
Preparation: Use the dry residue prepared as described in the assay.
Comparison: tylosin phosphate CRS.
B. Dilute a quantity of the bulk solution to be examined equivalent to 400 000 IU of tylosin phosphate to 10 mL with water R. The solution gives reaction (a) of phosphates (2.3.1).
TESTS
pH (2.2.3)
5.5 to 6.5.
Dilute 1.0 g of the bulk solution to be examined to 10 mL with carbon dioxide-free water R.
Composition
Liquid chromatography (2.2.29): use the normalisation procedure. Prepare the solutions immediately before use. Phosphate buffer pH 5.5 Dissolve 25.82 g of potassium dihydrogen phosphate R in 800 mL of water R, adjust to pH 5.5 with a 13.2 g/L solution of dipotassium hydrogen phosphate R and dilute to 1.0 L with water R.
Test solution: Dilute 1.0 g of the bulk solution to be examined to 50.0 mL with water R. Dilute 3.0 mL of this solution to 20.0 mL with water R (1.0 mg/mL of dried residue).
Reference solution (a): Dissolve 5 mg of tylosin for system suitability CRS (containing tylosins A, B, C and D, and impurities A, E, N, O, R and S) in 1 mL of acetonitrile R and dilute to 5.0 mL with water R.
Reference solution (b): Dilute 1.0 mL of the test solution to 100.0 mL with water R. Dilute 1.0 mL of this solution to 10.0 mL with water R.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 μm);
— temperature: 60 °C.
Mobile phase:
— mobile phase A: phosphate buffer pH 5.5, acetonitrile R, water for chromatography R (10:27.5:62.5 V/V/V);
— mobile phase B: phosphate buffer pH 5.5, water for chromatography R, acetonitrile R (10:40:50 V/V/V);
| Time
(min) |
Mobile phase A
(per cent V/V) |
Mobile phase B
(per cent V/V) |
| 0 – 25 | 100 | 0 |
| 25 – 45 | 100 → 84 | 0 → 16 |
| 45 – 65 | 84 | 16 |
| 65 – 70 | 84 → 44 | 16 → 56 |
| 70 – 82 | 44 | 56 |
Flow rate: 1.0 mL/min.
Detection: Spectrophotometer at 280 nm.
Injection: 20 μL.
Identification of peaks: Use the chromatogram supplied with tylosin for system suitability CRS and the chromatogram obtained with reference solution (a) to identify the peaks due to tylosins A, B, C and D, and impurities A, E, N, O, R and S.
Relative retention: With reference to tylosin A (retention time = about 65 min): impurity E = about 0.23; tylosin B = about 0.31; impurity A = about 0.38; tylosin C = about 0.60; tylosin D = about 0.78; impurity N = about 0.81; impurity O = about 0.85; impurity R = about 1.17; impurity S = about 1.20.
System suitability:
— resolution: reference solution (a): minimum 2.0 between the peaks due to tylosin B and impurity A; minimum 1.5 between the peaks due to impurities N and O; minimum 1.3 between the peaks due to impurities R and S;
— signal-to-noise ratio: minimum 10 for the principal peak in the chromatogram obtained with reference solution (b).
Limits:
— tylosin A: minimum 80.0 per cent;
— sum of tylosins A, B, C and D: minimum 95.0 per cent;
— reporting threshold: 0.10 per cent (reference solution (b)).
Related substances
Liquid chromatography (2.2.29) as described in the test for composition. Use the normalisation procedure.
Limits:
— impurity A: maximum 2.0 per cent;
— sum of impurities eluting between impurity A and tylosin C: maximum 2.0 per cent;
— impurities N, O: for each impurity, maximum 1.0 per cent;
— impurities E, R, S: for each impurity, maximum 0.5 per cent;
— any other impurity: for each impurity, maximum 0.50 per cent;
— total: maximum 5.0 per cent;
— reporting threshold: 0.10 per cent (reference solution (b)).
Impurity II (tyramine)
Maximum 0.35 per cent.
In a 25.0 mL volumetric flask, dilute a quantity of the bulk solution to be examined equivalent to 50 000 IU of tylosin phosphate to 5.0 mL with a 3.4 g/L solution of phosphoric acid R. Add 1.0 mL of pyridine R and 2.0 mL of a saturated solution of ninhydrin R (about 40 g/L). Close the flask with aluminium foil and heat in a water-bath at 85 °C for 20-30 min.
Cool the solution rapidly and dilute to 25.0 mL with water R. Mix and measure immediately the absorbance (2.2.25) of the solution at 570 nm using a blank solution as the compensation liquid. The absorbance is not greater than that of a standard prepared at the same time and in the same manner using 5.0 mL of a 35 mg/L solution of tyramine R (impurity II) in a 3.4 g/L solution of phosphoric acid R.
Phosphate
8.5 per cent to 10.0 per cent, calculated with reference to the dry residue (see Assay).
Test solution: Dilute a quantity of the bulk solution to be examined equivalent to 200 000 IU of tylosin phosphate to 50 mL with water R. Add 5.0 mL of dilute sulfuric acid R and dilute to 100.0 mL with water R. To 2.0 mL of this solution add successively, mixing after each addition, 10.0 mL of water R, 5.0 mL of ammonium molybdate reagent R2, 1.0 mL of hydroquinone solution R and 1.0 mL of a 200 g/L solution of sodium metabisulfite R. Allow to stand for at least 20 min and dilute to 50.0 mL with water R. Mix thoroughly.
Reference solution (a): To 1.0 mL of a standard solution containing 0.430 g/L of potassium dihydrogen phosphate R (corresponds to 300 ppm of PO4) add successively, mixing after each addition, 10.0 mL of water R, 5.0 mL of ammonium molybdate reagent R2, 1.0 mL of hydroquinone solution R and 1.0 mL of a 200 g/L solution of sodium metabisulfite R. Allow to stand for at least 20 min and dilute to 50.0 mL with water R. Mix thoroughly.
Reference solution (b): Prepare as reference solution (a) but using 2.0 mL of the standard solution.
Reference solution (c): Prepare as reference solution (a) but using 5.0 mL of the standard solution.
Compensation liquid: Prepare as reference solution (a) but omitting the standard solution.
Measure the absorbance (2.2.25) of the test solution and of the reference solutions at 650 nm. Draw a calibration curve with the absorbances of the 3 reference solutions as a function of the quantity of phosphate in the solutions and read from the curve the quantity of phosphate in the test solution. Determine the percentage content of phosphate, calculated with
reference to the dry residue (see Assay).
ASSAY
Carry out the microbiological assay of antibiotics (2.7.2).
Use tylosin CRS as the chemical reference substance. Calculate the content from the mass of the dry residue and the activity of the solution.
Dry residue Dry 3.0 g of the bulk solution to be examined in vacuo at 60 °C for 3 h and weigh.
STORAGE
Protected from light, at a temperature of 2 °C to 8 °C.
IMPURITIES
Specified impurities A, E, N, O, R, S, II.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities. It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) B, C, D, F, G, H, I, J, K, L, M, P, Q, T, U, V, W, X, Y, Z, AA, BB, CC, DD, EE, FF, GG, HH.
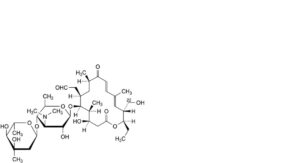
A. 22-O-demycinosyltylosin,
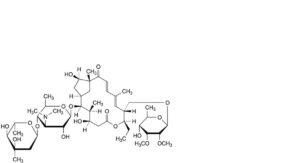
B. tylosin A aldol,
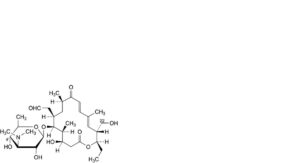
C. 4′-O-demycarosyl-22-O-demycinosyltylosin (demycinosyldesmycosin),
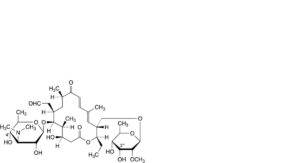
D. 3′′′-O-demethyl-4′-O-demycarosyltylosin (demycarosylmacrocin, lactenocin),
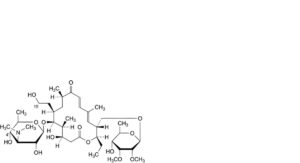
E. 4′-O-demycarosyl-19-deoxo-19-hydroxytylosin (demycarosylrelomycin),
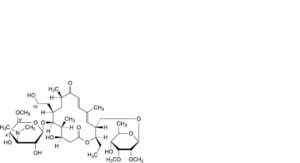
F. 5′-demethyl-4′-O-demycarosyl-19-deoxo-19-hydroxy-5′-methoxytylosin,
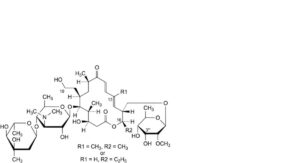
G. 16-deethyl-3′′′-O-demethyl-19-deoxo-19-hydroxy-16-methyltylosin or 3′′′-O,13-didemethyl-19-deoxo-19-hydroxytylosin,
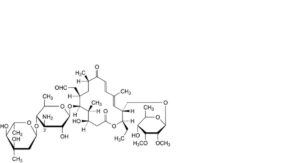
H. 3′-N,3′-N-didemethyltylosin,
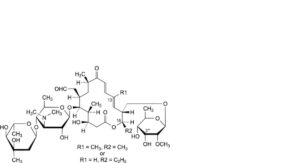
I. 16-deethyl-3′′′-O-demethyl-16-methyltylosin or 3′′′-O,13-didemethyltylosin,
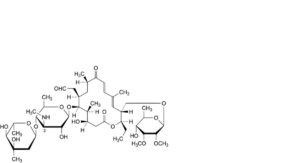
J. 3′-N-demethyltylosin,
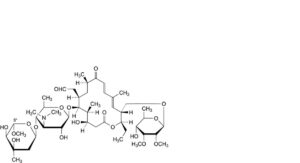
K. 5′′-demethyl-5′′-methoxytylosin,
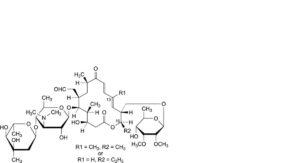
L. 16-deethyl-16-methyltylosin or 13-demethyltylosin,
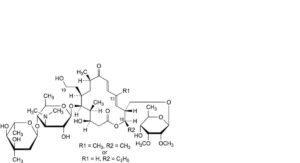
M. 16-deethyl-19-deoxo-19-hydroxy-16-methyltylosin or 13-demethyl-19-deoxo-19-hydroxytylosin,
N. unknown structure,
O. unknown structure,
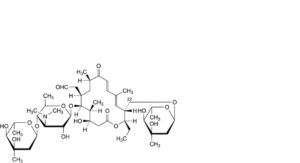
P. 22-O-demycinosyl-22-O-mycarosyltylosin,
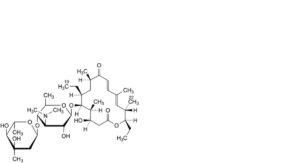
Q. 22-demycinosyloxy-19-deoxotylosin,
R. unknown structure,
S. unknown structure,
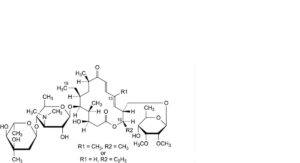
T. 16-deethyl-19-deoxo-16-methyltylosin or 13-demethyl-19-deoxotylosin,
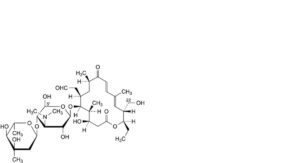
U. 5′-demethyl-22-O-demycinosyl-5′-hydroxytylosin,
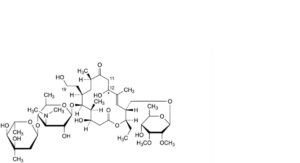
V. 19-deoxo-12,19-dihydroxy-11,12-dihydrotylosin,
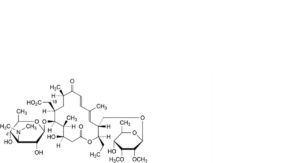
W. 18-carboxy-18-deformyl-4′-O-demycarosyltylosin,
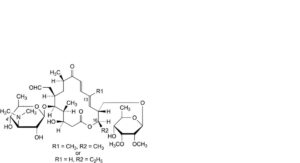
X. 16-deethyl-4′-O-demycarosyl-16-methyltylosin or 13-demethyl-4′-O-demycarosyltylosin,
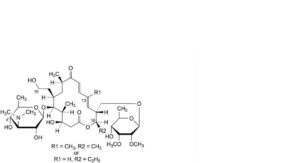
Y. 16-deethyl-4′-O-demycarosyl-19-deoxo-19-hydroxy-16-methyltylosin or 13-demethyl-4′-O-demycarosyl-19-deoxo-19-hydroxytylosin,
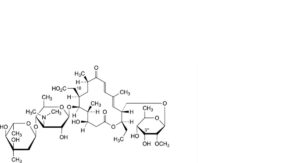
Z. 18-carboxy-18-deformyl-3′′′-O-demethyltylosin,
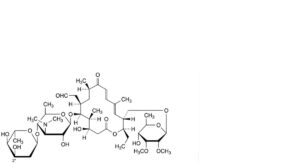
AA. 3′′-demethyltylosin,
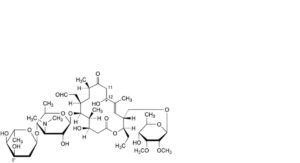
BB. 3′′-demethyl-12-hydroxy-11,12-dihydrotylosin,
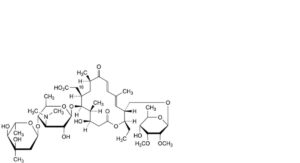
CC. 18-carboxy-18-deformyltylosin,
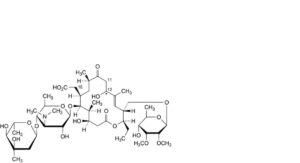
DD. 18-carboxy-18-deformyl-12-hydroxy-11,12-dihydrotylosin,
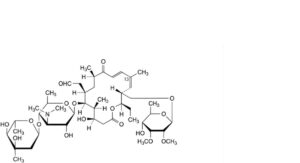
EE. (13Z)-tylosin,
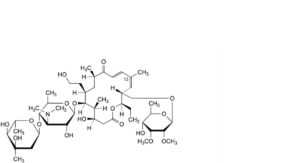
FF. (13Z)-19-deoxo-19-hydroxytylosin ((13Z)-relomycin),
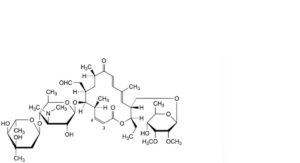
GG. 4-dehydroxy-3,4-didehydrotylosin,
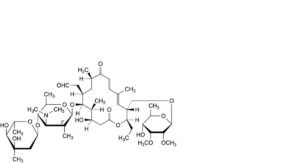
HH. 2′-deoxy-2′-methyltylosin,

II. 4-(2-aminoethyl)phenol (tyramine).