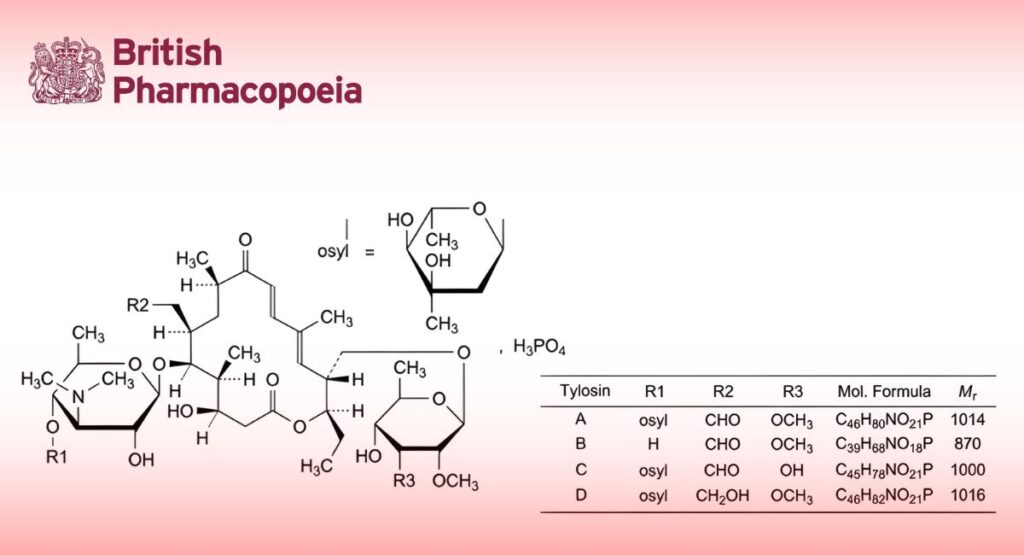
(Tylosin Phosphate for Veterinary Use, Ph. Eur. monograph 2802)
1405-53-4
Preparation
Tylosin Premix
DEFINITION
Dihydrogen phosphate of a mixture of macrolide antibiotics produced by certain strains of Streptomyces fradiae. The main component is 2-[(4R,5S,6S,7R,9R,11E,13E,15R,16R)-15-[[(6-deoxy-2,3-di-O-methyl-β-D-allopyranosyl)oxy]methyl]-6-[[3,6-dideoxy-4-O-(2,6-dideoxy-3-C-methyl-α-L-ribo-hexopyranosyl)-3-(dimethylamino)-β-D-glucopyranosyl]oxy]-16-ethyl-4-hydroxy-5,9,13-trimethyl-2,10-dioxo-1-oxacyclohexadeca-11,13-dien-7-yl]acetaldehyde monophosphate (tylosin monophosphate, tylosin A monophosphate). The monophosphates of tylosin B (desmycosin, 4′-O-demycarosyltylosin), tylosin C (macrocin, 3′′′-O-demethyltylosin) and tylosin D (relomycin, 19-deoxo-19-hydroxytylosin) may also be present.
Content
Minimum 900 IU/mg (dried substance). Tylosins A, B, C and D contribute to the potency.
CHARACTERS
Appearance
White or slightly yellow, hygroscopic powder.
Solubility
Freely soluble in water and in ethanol (96 per cent), practically insoluble in heptane.
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24).
Comparison: tylosin phosphate CRS.
B. Dissolve 50 mg in 10 mL of water R. The solution gives reaction (a) of phosphates (2.3.1).
TESTS
Appearance of solution
The solution is clear (2.2.1) and not more intensely coloured than reference solution Y3 (2.2.2, Method II).
Dissolve 1.00 g in 20 mL of a mixture of equal volumes of acetonitrile R and water R.
pH (2.2.3)
6.0 to 7.2.
Dissolve 0.25 g in 10 mL of carbon dioxide-free water R.
Composition
Liquid chromatography (2.2.29): use the normalisation procedure. Prepare the solutions immediately before use.
Phosphate buffer pH 5.5 Dissolve 25.82 g of potassium dihydrogen phosphate R in 800 mL of water R, adjust to pH 5.5 with a 13.2 g/L solution of dipotassium hydrogen phosphate R and dilute to 1.0 L with water R.
Test solution: Dissolve 25.0 mg of the substance to be examined in 5 mL of acetonitrile R and dilute to 25.0 mL with water R.
Reference solution (a): Dissolve 5 mg of tylosin for system suitability CRS (containing tylosins A, B, C and D, and impurities A, E, N, O, R and S) in 1 mL of acetonitrile R and dilute to 5.0 mL with water R.
Reference solution (b): Dilute 1.0 mL of the test solution to 100.0 mL with water R. Dilute 1.0 mL of this solution to 10.0 mL with water R.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 μm);
— temperature: 60 °C.
Mobile phase:
— mobile phase A: phosphate buffer pH 5.5, acetonitrile R, water for chromatography R (10:27.5:62.5 V/V/V);
— mobile phase B: phosphate buffer pH 5.5, water for chromatography R, acetonitrile R (10:40:50 V/V/V);
| Time
(min) |
Mobile phase A
(per cent V/V) |
Mobile phase B
(per cent V/V) |
| 0 – 25 | 100 | 0 |
| 25 – 45 | 100 → 84 | 0 → 16 |
| 45 – 65 | 84 | 16 |
| 65 – 70 | 84 → 44 | 16 → 56 |
| 70 – 82 | 44 | 56 |
Flow rate: 1.0 mL/min.
Detection: Spectrophotometer at 280 nm.
Injection: 20 μL.
Identification of peaks: Use the chromatogram supplied with tylosin for system suitability CRS and the chromatogram obtained with reference solution (a) to identify the peaks due to tylosins A, B, C and D, and impurities A, E, N, O, R and S.
Relative retention: With reference to tylosin A (retention time = about 65 min): impurity E = about 0.23; tylosin B = about 0.31; impurity A = about 0.38; tylosin C = about 0.60; tylosin D = about 0.78; impurity N = about 0.81; impurity O = about 0.85; impurity R = about 1.17; impurity S = about 1.20.
System suitability:
— resolution: reference solution (a): minimum 2.0 between the peaks due to tylosin B and impurity A; minimum 1.5 between the peaks due to impurities N and O; minimum 1.3 between the peaks due to impurities R and S;
— signal-to-noise ratio: minimum 10 for the principal peak in the chromatogram obtained with reference solution (b).
Limits:
— tylosin A: minimum 80.0 per cent;
— sum of tylosins A, B, C and D: minimum 95.0 per cent;
— reporting threshold: 0.10 per cent (reference solution (b)).
Related substances
Liquid chromatography (2.2.29) as described in the test for composition. Use the normalisation procedure.
Limits:
— impurity A: maximum 2.0 per cent;
— sum of impurities eluting between impurity A and tylosin C: maximum 2.0 per cent;
— impurities N, O: for each impurity, maximum 1.0 per cent;
— impurities E, R, S: for each impurity, maximum 0.5 per cent;
— any other impurity: for each impurity, maximum 0.50 per cent;
— total: maximum 5.0 per cent;
— reporting threshold: 0.10 per cent (reference solution (b)).
Impurity II (tyramine)
Maximum 0.35 per cent, or maximum 0.15 per cent if intended for use in the manufacture of parenteral preparations.
In a 25.0 mL volumetric flask, dissolve 50.0 mg in 5.0 mL of a 3.4 g/L solution of phosphoric acid R. Add 1.0 mL of pyridine R and 2.0 mL of a saturated solution of ninhydrin R (about 40 g/L). Close the flask with aluminium foil and heat in a water-bath at 85 °C for 20-30 min. Cool the solution rapidly and dilute to 25.0 mL with water R. Mix and measure immediately the absorbance (2.2.25) of the solution at 570 nm using a blank solution as the compensation liquid. The absorbance is not greater than that of a standard prepared at the same time and in the same manner using 5.0 mL of a 35 mg/L solution of tyramine R (impurity II) in a 3.4 g/L solution of phosphoric acid R. If intended for use in the manufacture of parenteral preparations, the absorbance is not greater than that of a standard prepared at the same time and in the same manner using 5.0 mL of a 15 mg/L solution of tyramine R (impurity II) in a 3.4 g/L solution of phosphoric acid R.
Loss on drying (2.2.32)
Maximum 4.5 per cent, determined on 1.000 g by drying in an oven at 60 °C at a pressure not exceeding 0.7 kPa for 3 h.
Sulfated ash (2.4.14)
Maximum 6.0 per cent, determined on 1.0 g.
ASSAY
Carry out the microbiological assay of antibiotics (2.7.2). Use tylosin CRS as the chemical reference substance.
STORAGE
In an airtight container, protected from light. If the substance is sterile, the container is also sterile and tamper-evident.
IMPURITIES
Specified impurities A, E, N, O, R, S, II.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities. It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) B, C, D, F, G, H, I, J, K, L, M, P, Q, T, U, V, W, X, Y, Z, AA, BB, CC, DD, EE, FF, GG, HH.
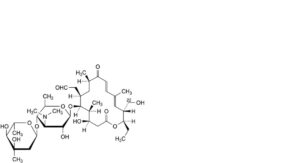
A. 22-O-demycinosyltylosin,
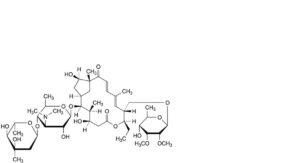
B. tylosin A aldol,
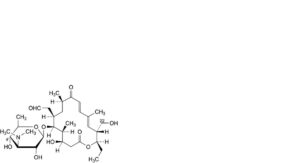
C. 4′-O-demycarosyl-22-O-demycinosyltylosin (demycinosyldesmycosin),
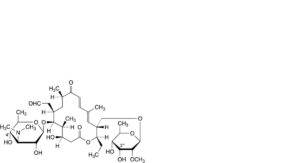
D. 3′′′-O-demethyl-4′-O-demycarosyltylosin (demycarosylmacrocin, lactenocin),
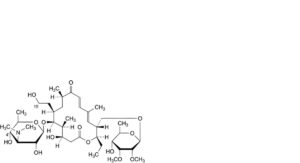
E. 4′-O-demycarosyl-19-deoxo-19-hydroxytylosin (demycarosylrelomycin),
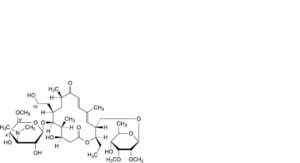
F. 5′-demethyl-4′-O-demycarosyl-19-deoxo-19-hydroxy-5′-methoxytylosin,
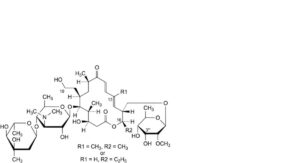
G. 16-deethyl-3′′′-O-demethyl-19-deoxo-19-hydroxy-16-methyltylosin or 3′′′-O,13-didemethyl-19-deoxo-19-hydroxytylosin,
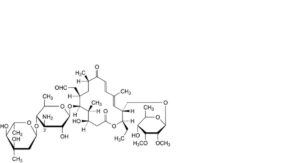
H. 3′-N,3′-N-didemethyltylosin,
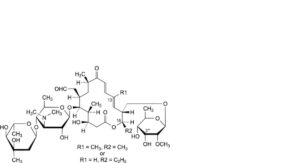
I. 16-deethyl-3′′′-O-demethyl-16-methyltylosin or 3′′′-O,13-didemethyltylosin,
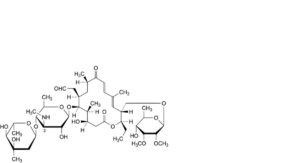
J. 3′-N-demethyltylosin,
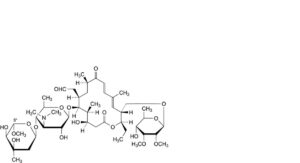
K. 5′′-demethyl-5′′-methoxytylosin,
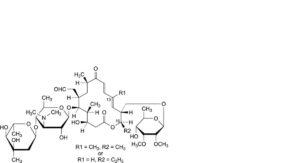
L. 16-deethyl-16-methyltylosin or 13-demethyltylosin,
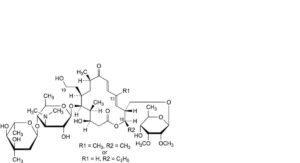
M. 16-deethyl-19-deoxo-19-hydroxy-16-methyltylosin or 13-demethyl-19-deoxo-19-hydroxytylosin,
N. unknown structure,
O. unknown structure,
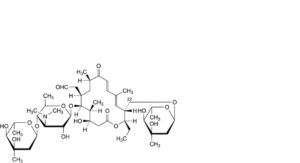
P. 22-O-demycinosyl-22-O-mycarosyltylosin,
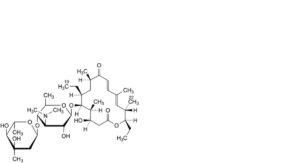
Q. 22-demycinosyloxy-19-deoxotylosin,
R. unknown structure,
S. unknown structure,
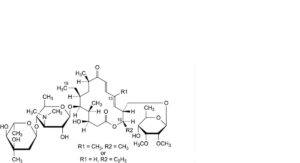
T. 16-deethyl-19-deoxo-16-methyltylosin or 13-demethyl-19-deoxotylosin,
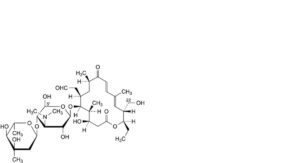
U. 5′-demethyl-22-O-demycinosyl-5′-hydroxytylosin,
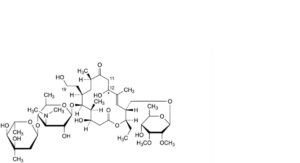
V. 19-deoxo-12,19-dihydroxy-11,12-dihydrotylosin,
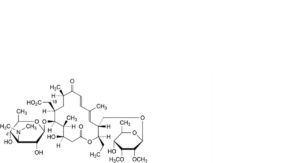
W. 18-carboxy-18-deformyl-4′-O-demycarosyltylosin,
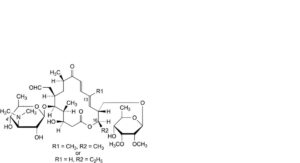
X. 16-deethyl-4′-O-demycarosyl-16-methyltylosin or 13-demethyl-4′-O-demycarosyltylosin,
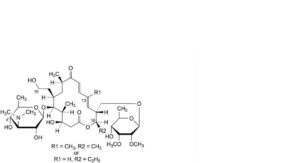
Y. 16-deethyl-4′-O-demycarosyl-19-deoxo-19-hydroxy-16-methyltylosin or 13-demethyl-4′-O-demycarosyl-19-deoxo-19-hydroxytylosin,
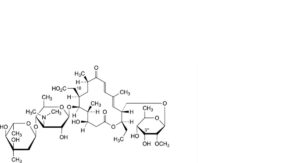
Z. 18-carboxy-18-deformyl-3′′′-O-demethyltylosin,
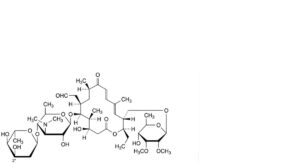
AA. 3′′-demethyltylosin,
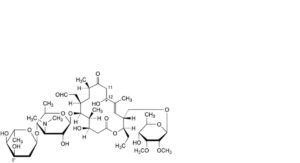
BB. 3′′-demethyl-12-hydroxy-11,12-dihydrotylosin,
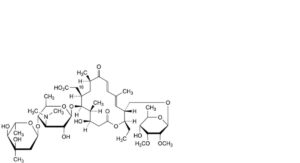
CC. 18-carboxy-18-deformyltylosin,
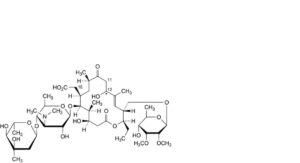
DD. 18-carboxy-18-deformyl-12-hydroxy-11,12-dihydrotylosin,
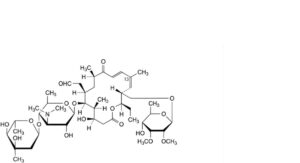
EE. (13Z)-tylosin,
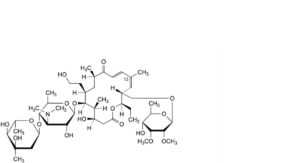
FF. (13Z)-19-deoxo-19-hydroxytylosin ((13Z)-relomycin),
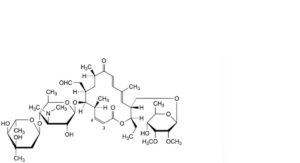
GG. 4-dehydroxy-3,4-didehydrotylosin,
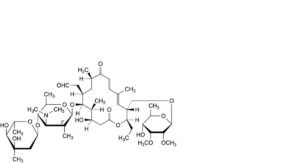
HH. 2′-deoxy-2′-methyltylosin,

II. 4-(2-aminoethyl)phenol (tyramine).