(Ph. Eur. 11.6 update)
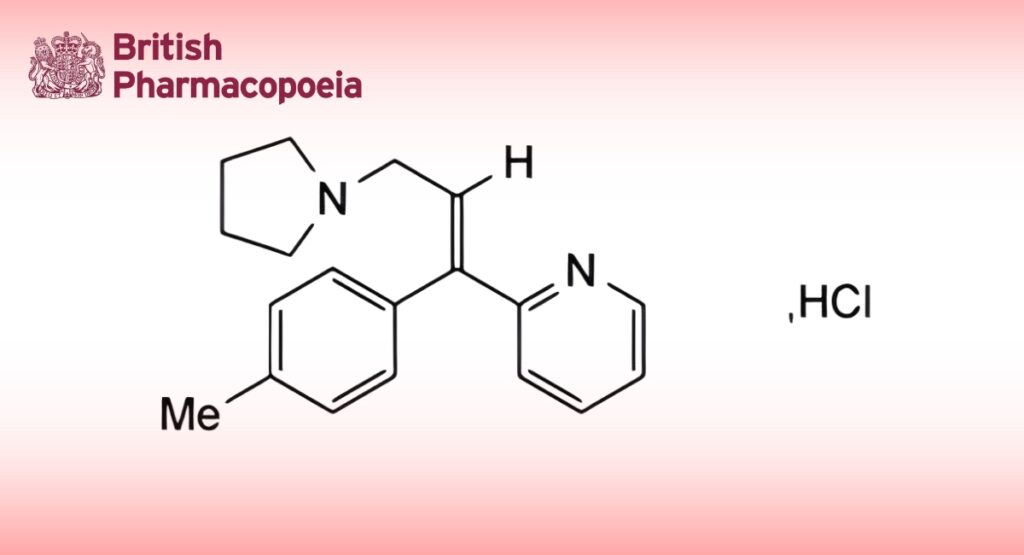
C19H22N2,HCl,H2O 332.9 6138-79-0
Action and use
Histamine H1 receptor antagonist; antihistamine.
Preparation
Triprolidine Tablets
DEFINITION
Triprolidine Hydrochloride is (E)-2-(3-pyrrolidin-1-yl-1-p-tolylprop1-enyl)pyridine hydrochloride monohydrate. It contains not less than 98.5% and not more than 101.0% of C19H22N2,HCl, calculated with reference to the anhydrous substance.
CHARACTERISTICS
A white, crystalline powder.
Freely soluble in water; freely soluble in ethanol (96%) and practically insoluble in ether.
IDENTIFICATION
A. The infrared absorption spectrum, Appendix II A, is concordant with the reference spectrum of triprolidine hydrochloride (RS 356).
B. Yields reaction A characteristic of chlorides, Appendix VI.
TESTS
Related substances
Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions in methanol.
(1) 1.0% w/v of the substance being examined.
(2) 0.020% w/v of Z-triprolidine hydrochloride BPCRS.
(3) 0.010% w/v of the substance being examined.
CHROMATOGRAPHIC CONDITIONS
(a) Use as the coating silica gel F254 (Merck silica gel 60 F254 plates are suitable).
(b) Use the mobile phase as described below.
(c) Apply 5 μL of each solution.
(d) Develop the plate to 15 cm.
(e) After removal of the plate, dry in air, examine under ultraviolet light (254 nm).
MOBILE PHASE
Equal volumes of butan-2-one and dimethylformamide.
LIMITS
In the chromatogram obtained with solution (1):
any spot corresponding to Z-triprolidine is not more intense than the spot in the chromatogram obtained with solution (2);
and any other secondary spot is not more intense than the spot in the chromatogram obtained with solution (3).
Sulfated ash
Not more than 0.1%, Appendix IX A.
Water
4.5 to 6.0% w/w, Appendix IX C. Use 0.4 g.
ASSAY
Carry out Method I for non-aqueous titration, Appendix VIII A, using 0.25 g dissolved in a mixture of 50 mL of anhydrous acetic acid and 0.5 mL of acetic anhydride and crystal violet solution as indicator. Each mL of 0.1M perchloric acid VS is equivalent to 15.74 mg of C19H22N2,HCl.