Edition: BP 2025 (Ph. Eur. 11.6 update)
General Notices
(Ph. Eur. monograph 0150)
TiO2 79.9 13463-67-7
Action and use
Protective; excipient.
Preparation
Titanium Ointment
Ph Eur
DEFINITION
Content
98.0 per cent to 100.5 per cent.
CHARACTERS
Appearance
White or almost white powder.
Solubility
Practically insoluble in water. It does not dissolve in dilute mineral acids but dissolves slowly in hot concentrated sulfuric acid.
IDENTIFICATION
A. When strongly heated, it becomes pale yellow; the colour disappears on cooling.
B. To 5 mL of solution S (see Tests) add 0.1 mL of strong hydrogen peroxide solution R. An orange-red colour appears.
C. To 5 mL of solution S add 0.5 g of zinc R in granules. After 45 min, the mixture has a violet-blue colour.
TESTS
Solution S
Mix 0.500 g (m g) and 5 g of anhydrous sodium sulfate R in a 300 mL long-necked combustion flask. Add 10 mL of water R and mix. Add 10 mL of sulfuric acid R and boil vigorously, with the usual precautions, until a clear solution is obtained. Cool, add slowly a cooled mixture of 30 mL of water R and 10 mL of sulfuric acid R, cool again and dilute to 100.0 mL with water R.
Appearance of solution
Solution S is not more opalescent than reference suspension II (2.2.1) and is colourless (2.2.2, Method II).
Acidity or alkalinity
Shake 5.0 g with 50 mL of carbon dioxide-free water R for 5 min. Centrifuge or filter until a clear solution is obtained. To 10 mL of the solution add 0.1 mL of bromothymol blue solution R1. Not more than 1.0 mL of 0.01 M hydrochloric acid or 0.01 M sodium hydroxide is required to change the colour of the indicator.
Water-soluble substances
Maximum 0.5 per cent.
To 10.0 g add a solution of 0.5 g of ammonium sulfate R in 150 mL of water R and boil for 5 min. Cool, dilute to 200 mL with water R and filter until a clear solution is obtained. Evaporate 100 mL of the solution to dryness in a tared evaporating dish and ignite. The residue weighs a maximum of 25 mg.
Elemental impurities
Any method that fulfils the requirements of general chapter 2.4.20. Determination of elemental impurities may be used.
| Element | Maximum content (ppm) |
| Antimony
Arsenic Barium Lead |
20
1 20 5 |
Iron
Maximum 200 ppm.
To 8 mL of solution S add 4 mL of water R. Mix and add 0.05 mL of bromine water R. Allow to stand for 5 min and remove the excess of bromine with a current of air. Add 3 mL of potassium thiocyanate solution R. Any colour in the solution is not more intense than that in a standard prepared at the same time in the same manner using a mixture of 4 mL of iron standard solution (2 ppm Fe) R and 8 mL of a 200 g/L solution of sulfuric acid R.
ASSAY
To 300 g of zinc R in granules (710) (2.1.4) add 300 mL of a 20 g/L solution of mercuric nitrate R and 2 mL of nitric acid R, shake for 10 min and wash with water R. Pack the amalgamated zinc into a glass tube about 400 mm long and about 20 mm in diameter fitted with a tap and a filter plate. Pass through the column 100 mL of dilute sulfuric acid R followed by 100 mL of water R, making sure that the amalgam is always covered with liquid. Pass slowly at a rate of about 3 mL/min through the column a mixture of 100 mL of dilute sulfuric acid R and 100 mL of water R followed by 100 mL of water R. Collect the eluate in a 500 mL conical flask containing 50.0 mL of a 150 g/L solution of ferric ammonium sulfate R in a mixture of 1 volume of sulfuric acid R and 3 volumes of water R. Add 0.1 mL of ferroin R and titrate immediately with 0.1 M ammonium and cerium nitrate until a greenish colour is obtained (n1 mL). Pass slowly at a rate of about 3 mL/min through the column a mixture of 50 mL of dilute sulfuric acid R and 50 mL of water R, followed by 20.0 mL of solution S, a mixture of 50 mL of dilute sulfuric acid R and 50 mL of water R and finally 100 mL of water R. Collect the eluate in a 500 mL conical flask containing 50.0 mL of a 150 g/L solution of ferric ammonium sulfate R in a mixture of 1 volume of sulfuric acid R and 3 volumes of water R. Rinse the lower end of the column with water R, add 0.1 mL of ferroin R and titrate immediately with 0.1 M ammonium and cerium nitrate until a greenish colour is obtained (n2 mL).
Calculate the percentage content of TiO2 using the following expression:
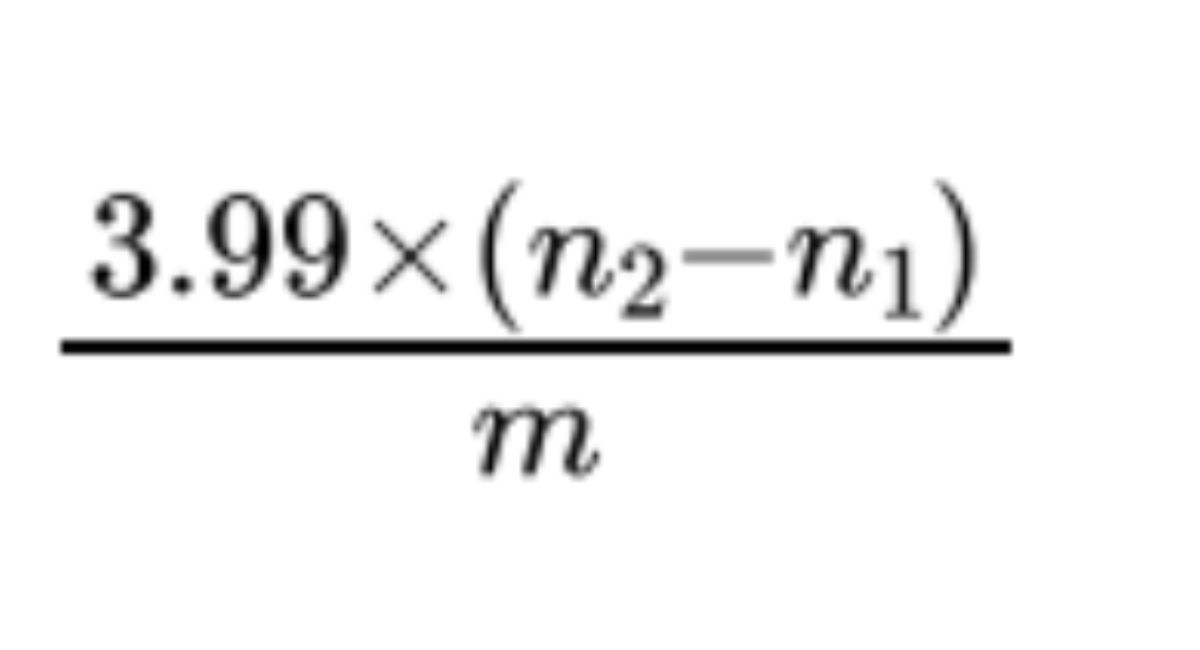
m = mass of the substance to be examined used for the preparation of solution S, in grams.
FUNCTIONALITY-RELATED CHARACTERISTICS
This section provides information on characteristics that are recognised as being relevant control parameters for one or more functions of the substance when used as an excipient (see chapter 5.15). Some of the characteristics described in the Functionality-related characteristics section may also be present in the mandatory part of the monograph since they also represent mandatory quality criteria. In such cases, a cross-reference to the tests described in the mandatory part is included in the Functionality-related characteristics section. Control of the characteristics can contribute to the quality of a medicinal product by improving the consistency of the manufacturing process and the performance of the medicinal product during use. Where control methods are cited, they are recognised as being suitable for the purpose, but other methods can also be used. Wherever results for a particular characteristic are reported, the control method must be indicated.
The following characteristic may be relevant for titanium dioxide used as opacifier in solid oral dosage forms and in preparations for cutaneous application.
Particle-size distribution (2.9.31)
Ph Eur






