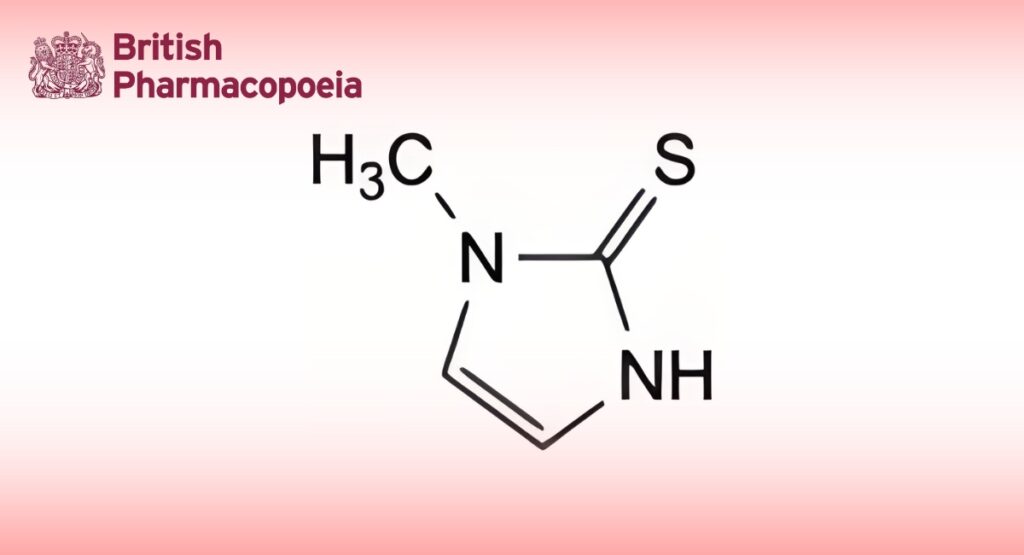
(Ph. Eur. monograph 1706)
C4H6N2S 114.2 60-56-0
Action and use
Thionamide antithyroid.
DEFINITION
1-Methyl-1,3-dihydro-2H-imidazole-2-thione.
Content
98.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or pale brown, crystalline powder.
Solubility
Freely soluble in water, freely soluble in methylene chloride, freely soluble or soluble in ethanol (96 per cent).
IDENTIFICATION
First identification: A, C.
Second identification: A, B, D.
A. Melting point (2.2.14): 143 °C to 146 °C.
B. Dissolve 25 mg in 10 mL of a 0.28 per cent V/V solution of sulfuric acid R and dilute to 50.0 mL with the same solution. Dilute 1.0 mL of this solution to 100.0 mL with a 0.28 per cent V/V solution of sulfuric acid R. Examined between 200 nm and 300 nm (2.2.25), the solution shows 2 absorption maxima, at 211 nm and 251 nm. The ratio of the absorbance measured at the absorption maximum at 251 nm to that measured at the absorption maximum at 211 nm is 2.5 to 2.7.
C. Infrared absorption spectrophotometry (2.2.24).
Preparation Discs.
Comparison thiamazole CRS.
D. Thin-layer chromatography (2.2.27).
Test solution Dissolve 5.0 mg of the substance to be examined in methanol R and dilute to 5.0 mL with the same solvent.
Reference solution (a) Dissolve 5.0 mg of thiamazole CRS in methanol R and dilute to 5.0 mL with the same solvent.
Reference solution (b) Dissolve 5.0 mg of 2-methylimidazole R in methanol R and dilute to 5.0 mL with the same solvent. Dilute 1.0 mL of this solution to 2.0 mL with the test solution.
Plate TLC silica gel F254 plate R.
Mobile phase concentrated ammonia R1, 2-propanol R, toluene R (1:24:75 V/V/V).
Application 10 μL.
Development Over 2/3 of the plate.
Drying In air.
Detection Examine in ultraviolet light at 254 nm.
System suitability Reference solution (b):
— expose the plate to iodine vapour for 30 min; the chromatogram shows 2 clearly separated spots.
Results The principal spot in the chromatogram obtained with the test solution is similar in position and size to the principal spot in the chromatogram obtained with reference solution (a).
TESTS
Solution S
Dissolve 2.0 g in water R and dilute to 20.0 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and not more intensely coloured than reference solution B6 (2.2.2, Method II).
Related substances
Gas chromatography (2.2.28).
Test solution Dissolve 0.100 g of the substance to be examined in chloroform R and dilute to 10.0 mL with the same solvent.
Reference solution (a) Dilute 1.0 mL of the test solution to 100.0 mL with chloroform R. Dilute 1.0 mL of this solution to 10.0 mL with chloroform R.
Reference solution (b) Dissolve 5.0 mg of thiamazole impurity A CRS, 5.0 mg of 1-methylimidazole R1 and 5.0 mg of thiamazole impurity C CRS in chloroform R and dilute to 50.0 mL with the same solvent. Dilute 1.0 mL of this solution to 10.0 mL with chloroform R.
Column:
— material: fused silica,
— size: l = 30.0 m, Ø = 0.25 mm,
— stationary phase: base-deactivated phenyl(5)methyl(95)polysiloxane R (film thickness 0.5 μm).
Carrier gas helium for chromatography R.
Flow rate 1.5 mL/min.
Split ratio 3:20.
Temperature:
| Time (min) | Temperature (°C) | |
| Column | 0 – 2
2 – 7 7 -22 |
100
100 → 250 250 |
| Injection port | 150 | |
| Detector | 250 |
Detection Flame ionisation.
Injection 1 μL.
Relative retention With reference to thiamazole (retention time = about 6.5 min): impurity A = about 0.3; impurity B = about 0.4; impurity C = about 0.7.
System suitability Reference solution (b):
— resolution: minimum 1.5 between the peaks due to impurity A and impurity B.
Limits:
— impurities A, B, C: for each impurity, not more than the area of the corresponding peak in the chromatogram obtained with reference solution (b) (0.1 per cent),
— any other impurity: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.1 per cent),
— total: not more than 5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.5 per cent),
— disregard limit: 0.2 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.02 per cent).
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C for 2 h.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.250 g in 75 mL of water R. Add 15.0 mL of 0.1 M sodium hydroxide, mix and add with stirring, about 30 mL of 0.1 M silver nitrate. Continue the titration with 0.1 M sodium hydroxide, determining the end point potentiometrically (2.2.20).
1 mL of 0.1 M sodium hydroxide is equivalent to 11.42 mg of C4H6N2S.
IMPURITIES
Specified impurities A, B, C.
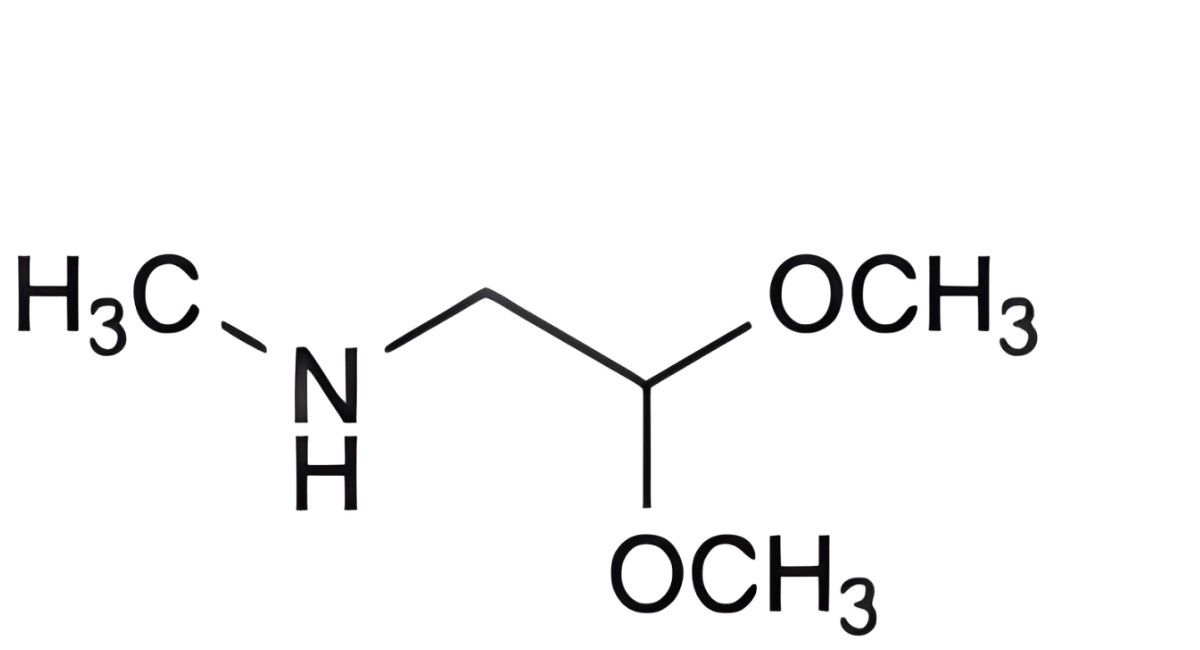
A. 2,2-dimethoxy-N-methylethanamine,
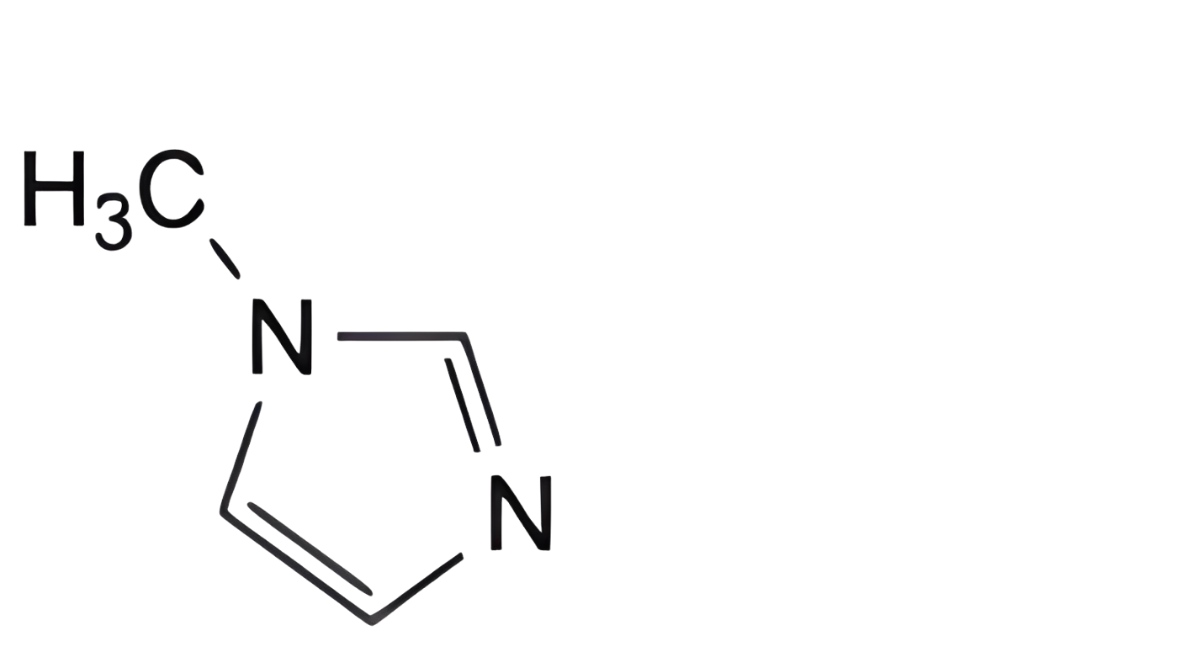
B. 1-methyl-1H-imidazole,
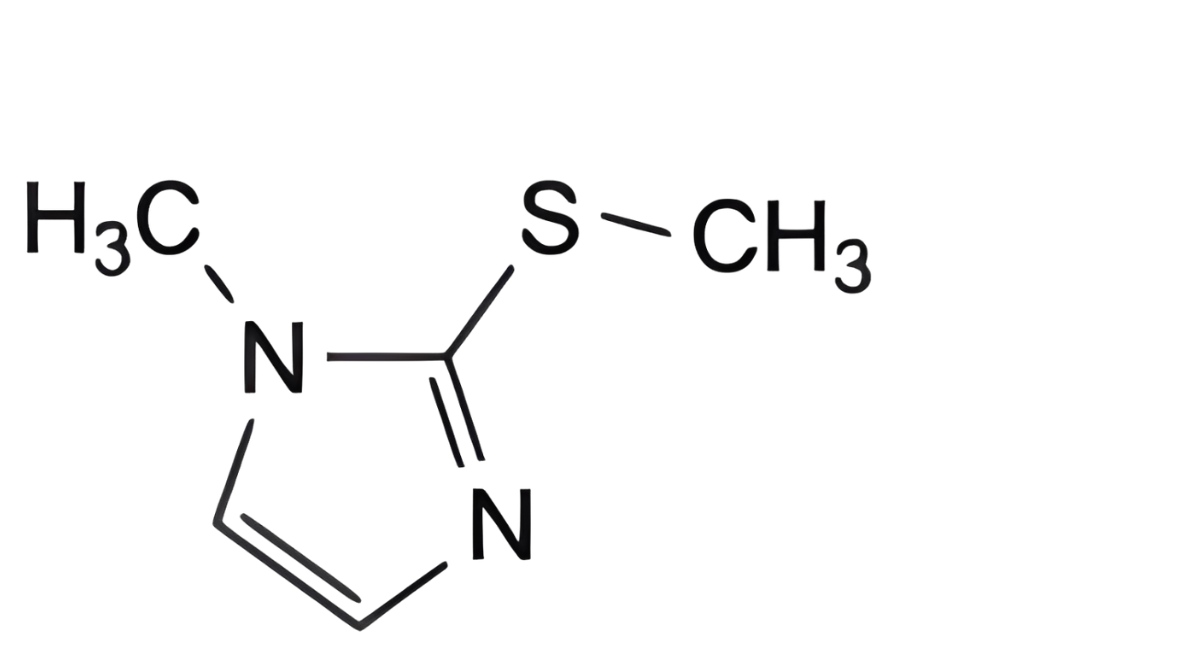
C. 1-methyl-2-(methylsulfanyl)-1H-imidazole.