BP 2025 (Ph. Eur. 11.6 update)
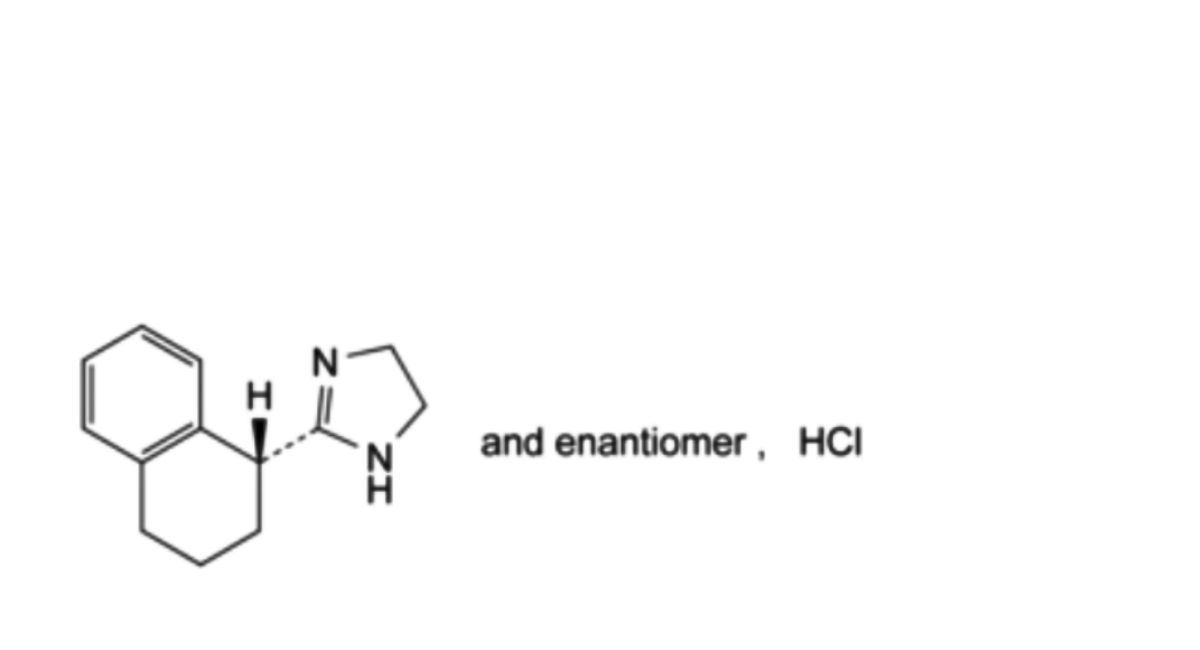
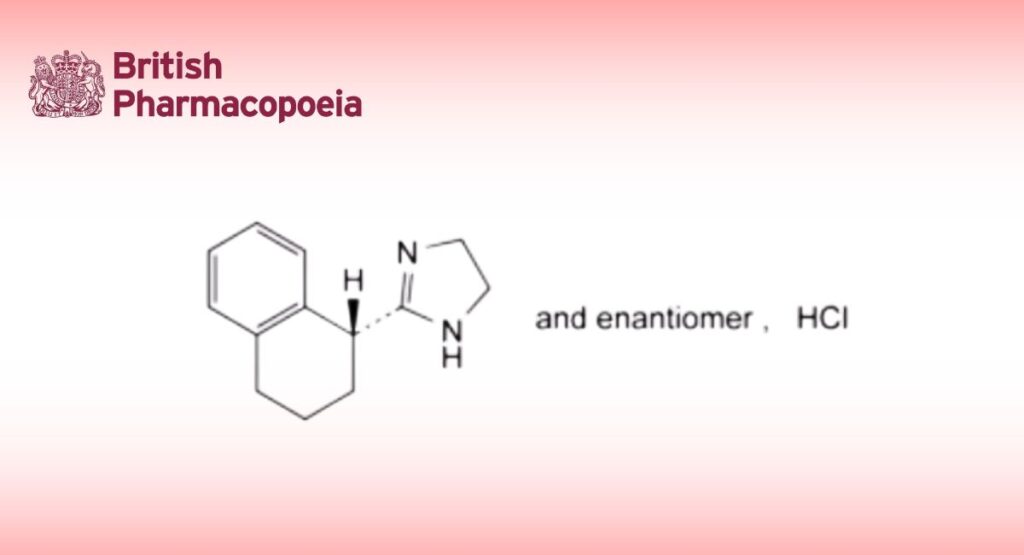
C13H17ClN2 236.7 522-48-5
Action and use
Adrenoceptor agonist; decongestant.
DEFINITION
2-[(1RS)-1,2,3,4-Tetrahydronaphthalen-1-yl]-4,5-dihydro-1H-imidazole hydrochloride.
Content
98.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Freely soluble in water, in anhydrous ethanol and in ethanol (96 per cent), practically insoluble in acetone.
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24).
Comparison tetryzoline hydrochloride CRS.
B. Dissolve 50 mg in 10 mL of water R. The solution gives reaction (a) of chlorides (2.3.1).
TESTS
Appearance of solution
The solution is clear (2.2.1) and colourless (2.2.2, Method II).
Dissolve 1.0 g in water R and dilute to 10 mL with the same solvent.
Related substances
Gas chromatography (2.2.28).
Test solution Dissolve 1.0 g of the substance to be examined in a mixture of 25 volumes of 1 M sodium hydroxide and
75 volumes of methanol R and dilute to 10 mL with the same mixture of solvents.
Reference solution Dilute 1.0 mL of the test solution to 100.0 mL with a mixture of 25 volumes of 1 M sodium hydroxide and 75 volumes of methanol R. Dilute 1.0 mL of this solution to 10.0 mL with a mixture of 25 volumes of 1 M sodium hydroxide and 75 volumes of methanol R.
Column:
— material: fused silica,
— size: l = 25 m, Ø = 0.32 mm,
— stationary phase: methylpolysiloxane R (1 μm).
Carrier gas helium for chromatography R.
Split ratio 1:40.
Flow rate 2.5 mL/min.
Temperature:
| Time (min) |
Temperature (°C) |
|
| Column | 0 – 8 | 160 |
| 8 – 11 | 160 → 220 | |
| 11 – 15 | 220 | |
| Injection port | 220 | |
| Detector | 220 |
Detection Flame ionisation.
Injection 1 μL.
Relative retention With reference to tetryzoline (retention time = about 12 min): impurity A = about 0.5.
System suitability Reference solution:
— signal-to-noise ratio: minimum 50 for the principal peak.
Limits:
— impurity A: not more than the area of the principal peak in the chromatogram obtained with the reference solution (0.1 per cent),
— any other impurity: for each impurity, not more than the area of the principal peak in the chromatogram obtained with the reference solution (0.1 per cent),
— total: not more than twice the area of the principal peak in the chromatogram obtained with the reference solution (0.2 per cent),
— disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with the reference solution (0.05 per cent).
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.200 g in 100 mL of a mixture of 3 volumes of anhydrous acetic acid R and 7 volumes of acetic anhydride R.
Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20). Carry out a blank titration.
1 mL of 0.1 M perchloric acid is equivalent to 23.67 mg of C13H17ClN2
IMPURITIES
Specified impurities A.
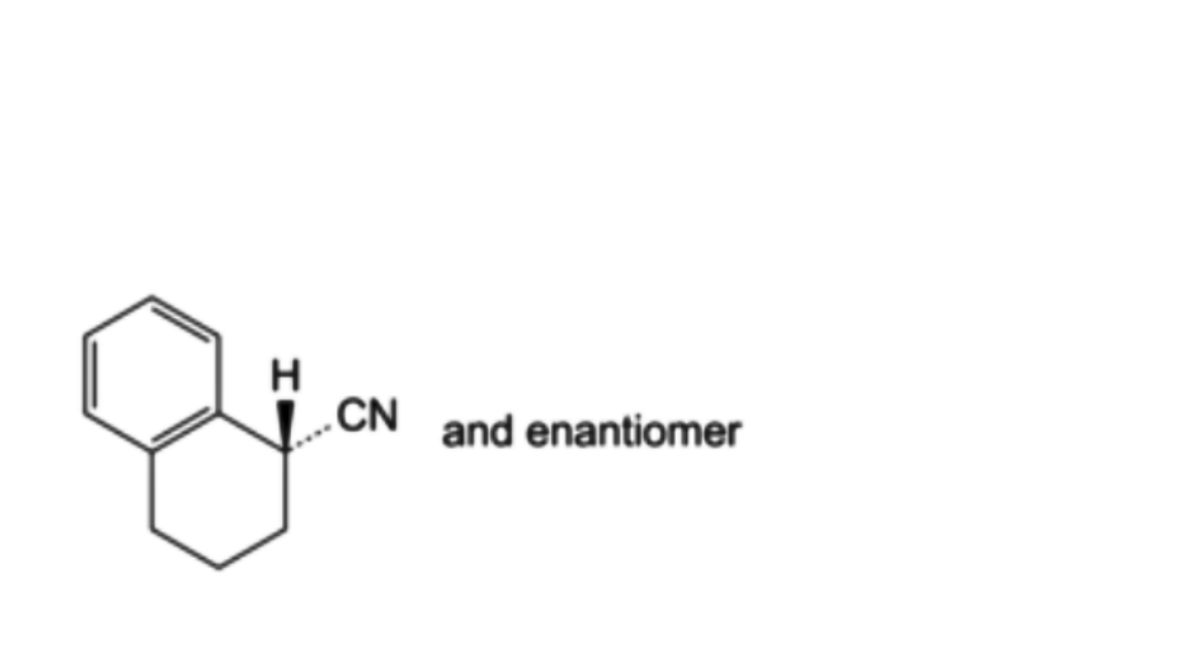
A. (1RS)-1,2,3,4-tetrahydronaphthalene-1-carbonitrile (α-cyanotetraline).