Edition: BP 2025 (Ph. Eur. 11.6 update)
(Ph. Eur. monograph 1737)
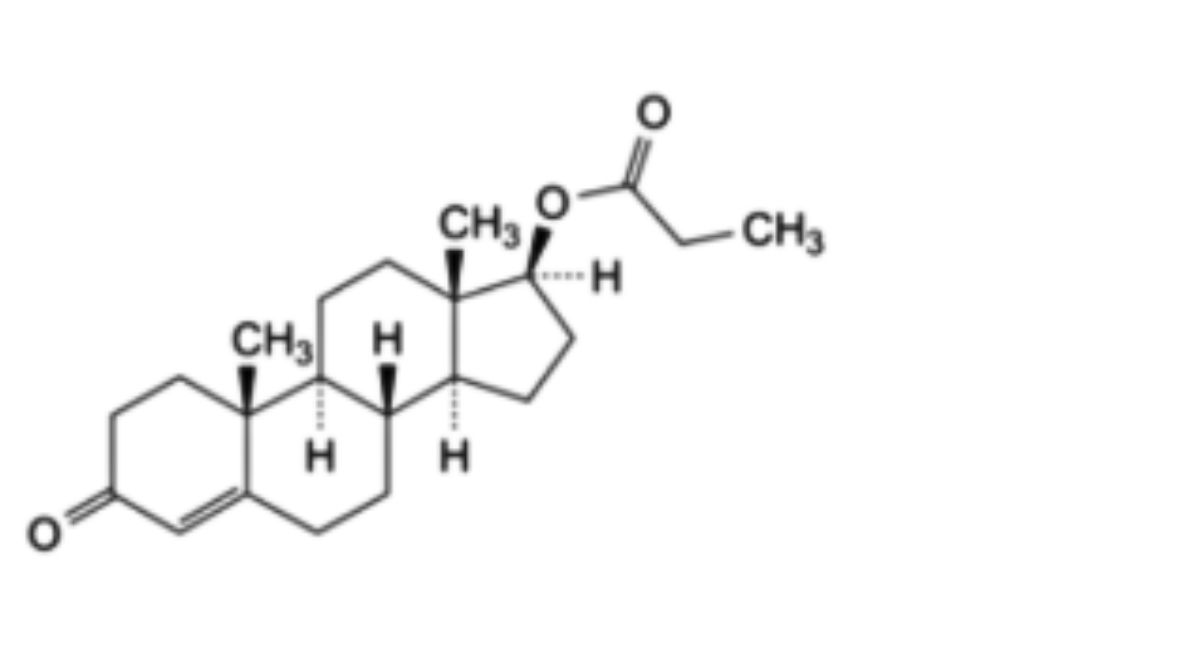
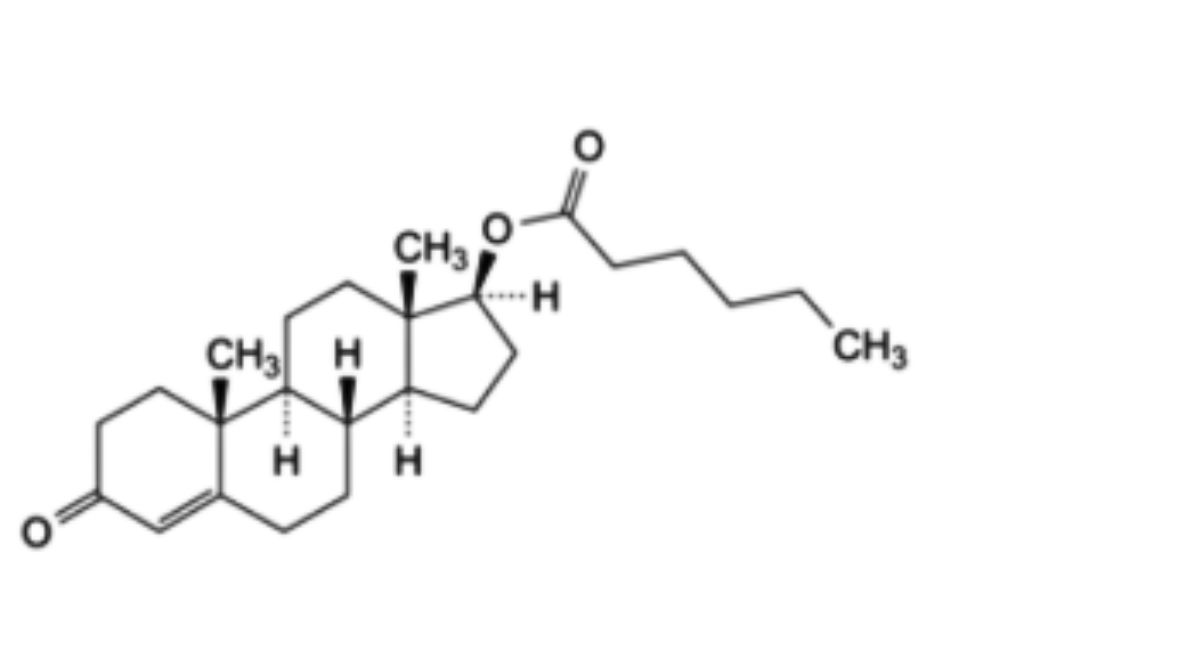
C₂₅H₃₈O₃ 386.6
Action and use
Androgen.
Ph Eur
DEFINITION
3-Oxoandrost-4-en-17β-yl 4-methylpentanoate.
Content
97.0 per cent to 102.0 per cent (dried substance).
CHARACTERS
Appearance:
White or almost white powder.
Solubility:
Practically insoluble in water, very soluble in acetone and in methylene chloride, freely soluble in fatty oils.
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24).
Comparison testosterone isocaproate CRS.
B. Examine the chromatograms obtained in the assay.
Results The principal peak in the chromatogram obtained with the test solution is similar in retention time and size to the principal peak in the chromatogram obtained with reference solution (c).
TESTS
Appearance of solution
The solution is clear (2.2.1) and not more intensely coloured than reference solution BY6 (2.2.2, Method II).
Dissolve 0.20 g in 20 mL of methanol R.
Specific optical rotation (2.2.7)
+82.0 to +88.0 (dried substance).
Dissolve 0.200 g in anhydrous ethanol R and dilute to 20.0 mL with the same solvent.
Related substances
Liquid chromatography (2.2.29).
Test solution:
Dissolve 20.0 mg of the substance to be examined in the mobile phase and dilute to 100.0 mL with the mobile phase.
Reference solution (a):
Dissolve 2 mg of testosterone isocaproate for system suitability CRS (containing impurities A, B, C, D, E, F and G) in 10 mL of the mobile phase.
Reference solution (b):
Dilute 10.0 mL of the test solution to 100.0 mL with the mobile phase. Dilute 1.0 mL of this solution to 20.0 mL with the mobile phase.
Reference solution (c):
Dissolve 20.0 mg of testosterone isocaproate CRS in the mobile phase and dilute to 100.0 mL with the mobile phase.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 µm);
— temperature: 40 °C.
Mobile phase:
water R, acetonitrile R (15:85 V/V).
Flow rate:
1.0 mL/min.
Detection:
Spectrophotometer at 240 nm.
Injection:
20 µL of the test solution and reference solutions (a) and (b).
Run time:
Twice the retention time of testosterone isocaproate.
Identification of impurities:
Use the chromatogram supplied with testosterone isocaproate for system suitability CRS and the chromatogram obtained with reference solution (a) to identify the peaks due to impurities A, B, C, D, E, F and G.
Relative retention:
With reference to testosterone isocaproate (retention time = about 14 min):
impurity A = about 0.2;
impurity B = about 0.4;
impurity C = about 0.5;
impurity D = about 0.7;
impurity G = about 0.8;
impurity E = about 1.1;
impurity F = about 1.4.
System suitability:
Reference solution (a):
— peak-to-valley ratio: minimum 2.5, where Hp = height above the baseline of the peak due to impurity E and Hv = height above the baseline of the lowest point of the curve separating this peak from the peak due to testosterone isocaproate.
Limits:
— impurities A, B, C, D, E, F, G: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (0.5 per cent);
— unspecified impurities: for each impurity, not more than 0.2 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.10 per cent);
— total: not more than twice the area of the principal peak in the chromatogram obtained with reference solution (b) (1.0 per cent);
— disregard limit: 0.1 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 per cent).
Free acid
Dissolve 0.44 g in 10 mL of ethanol (96 per cent) R, previously neutralised to bromothymol blue solution R3, and titrate immediately with 0.01 M sodium hydroxide, using 0.1 mL of bromothymol blue solution R3 as indicator.
Not more than 0.6 mL of 0.01 M sodium hydroxide is required to change the colour of the indicator to blue.
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in vacuo at a pressure not exceeding 0.7 kPa.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modification.
Injection:
20 µL of the test solution and reference solution (c).
Calculate the percentage content of C₂₅H₃₈O₃ from the declared content of testosterone isocaproate CRS.
IMPURITIES
Specified impurities:
A, B, C, D, E, F, G.