Edition: BP 2025 (Ph. Eur. 11.6 update)
General Notices
(Ph. Eur. monograph 1048)
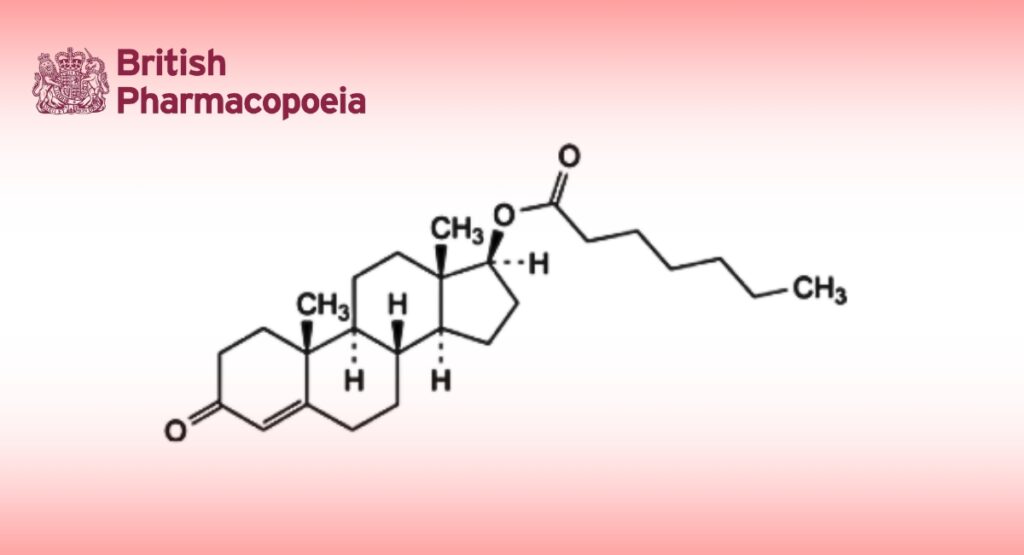
C26H40O3 400.6 315-37-7
Action and use
Androgen.
Ph Eur
DEFINITION
3-Oxoandrost-4-en-17β-yl heptanoate.
Content
97.0 per cent to 103.0 per cent (dried substance).
CHARACTERS
Appearance
White or yellowish-white, crystalline powder.
Solubility
Practically insoluble in water, very soluble in anhydrous ethanol, freely soluble in fatty oils.
IDENTIFICATION
First identification: B.
Second identification: A, C, D.
A. Melting point (2.2.14): 34 °C to 39 °C.
B. Infrared absorption spectrophotometry (2.2.24).
Comparison testosterone enantate CRS.
C. Thin-layer chromatography (2.2.27).
Solvent mixture methanol R, methylene chloride R (10:90 V/V).
Test solution Dissolve 5 mg of the substance to be examined in the solvent mixture and dilute to 10 mL with the solvent mixture.
Reference solution (a) Dissolve 5 mg of testosterone enantate CRS in the solvent mixture and dilute to 10 mL with the solvent mixture.
Reference solution (b) Dissolve 5 mg of testosterone enantate CRS, 5 mg of testosterone decanoate CRS and 5 mg of testosterone isocaproate CRS in the solvent mixture and dilute to 10 mL with the solvent mixture.
Plate TLC octadecylsilyl silica gel F254 plate R.
Mobile phase water R, acetonitrile R, 2-propanol R (20:40:60 V/V/V).
Application 5 μL.
Development Over 3/4 of the plate.
Drying In air, then at 100 °C for 10 min; allow to cool.
Detection A Examine in ultraviolet light at 254 nm.
Results A The principal spot in the chromatogram obtained with the test solution is similar in position and size to the principal spot in the chromatogram obtained with reference solution (a).
Detection B Spray with alcoholic solution of sulfuric acid R; heat at 120 °C for 10 min; allow to cool and examine in daylight.
Results B The principal spot in the chromatogram obtained with the test solution is green and is similar in position and size to the principal spot in the chromatogram obtained with reference solution (a).
System suitability Reference solution (b):
— the chromatogram shows 3 clearly separated principal spots by each method of visualisation.
D. To about 25 mg add 2 mL of a 10 g/L solution of potassium hydroxide R in methanol R and boil under a reflux condenser for 1 h. Cool. Add 10 mL of water R. Acidify with dilute hydrochloric acid R until blue litmus paper R turns red.
Filter and wash the precipitate with a small quantity of water R. The residue, after drying at 60 °C at a pressure not exceeding 0.7 kPa for 3 h, melts (2.2.14) at 150 °C to 153 °C.
TESTS
Specific optical rotation (2.2.7)
+ 81 to + 86 (dried substance).
Dissolve 0.100 g in anhydrous ethanol R and dilute to 10.0 mL with the same solvent.
Impurity A
Maximum 0.16 per cent.
Dissolve 0.50 g in 10 mL of ethanol (96 per cent) R previously neutralised to bromothymol blue solution R3. Titrate
immediately with 0.01 M sodium hydroxide using 0.1 mL of bromothymol blue solution R3 as indicator. Not more than 0.6 mL of 0.01 M sodium hydroxide is required to change the colour of the indicator to blue.
Impurity H
Thin-layer chromatography (2.2.27).
Test solution Dissolve 0.100 g of the substance to be examined in 1.0 mL of ethanol (96 per cent) R.
Reference solution Dissolve 3.0 mg of testosterone enantate impurity H CRS in 20.0 mL of ethanol (96 per cent) R.
Plate TLC silica gel plate R.
Mobile phase ethyl acetate R, cyclohexane R1 (40:60 V/V).
Application 1 μL.
Development Over 2/3 of the plate.
Drying In air.
Detection Spray with a 200 g/L solution of toluenesulfonic acid R in ethanol (96 per cent) R and heat at 120 °C for 10 min; examine in ultraviolet light at 366 nm.
System suitability Reference solution:
— the chromatogram shows a clearly visible spot due to impurity H.
Limit:
— impurity H: any spot due to impurity H is not more intense than the principal spot in the chromatogram obtained with the reference solution (0.15 per cent).
Related substances
Liquid chromatography (2.2.29).
Test solution Dissolve 20 mg of the substance to be examined in the mobile phase and dilute to 10.0 mL with the mobile phase.
Reference solution (a) Dissolve with the aid of ultrasound the contents of a vial of testosterone enantate for system suitability CRS (containing impurities F and G) in the mobile phase and dilute to 1.0 mL with the mobile phase.
Reference solution (b) Dilute 2.0 mL of the test solution to 100.0 mL with the mobile phase. Dilute 1.0 mL of this solution to 20.0 mL with the mobile phase.
Reference solution (c) Dissolve 2 mg of testosterone enantate for peak identification CRS (containing impurity E) in 1.0 mL of the mobile phase.
Reference solution (d) Dissolve 2 mg of testosterone caproate CRS (impurity B) and 2 mg of testosterone CRS (impurity D) in the mobile phase and dilute to 5.0 mL with the mobile phase. Dilute 1.0 mL of the solution to 100.0 mL with the mobile phase.
Column:
— size: l = 0.15 m, Ø = 4.6 mm;
— stationary phase: spherical end-capped dodecylsilyl silica gel for chromatography R (4 μm).
Mobile phase water R, acetonitrile R (30:70 V/V).
Flow rate 2.0 mL/min.
Detection Spectrophotometer at 242 nm.
Injection 10 μL.
Run time 1.5 times the retention time of testosterone enantate.
Identification of impurities Use the chromatogram supplied with testosterone enantate for system suitability CRS and the chromatogram obtained with reference solution (a) to identify the peaks due to impurities F and G; use the chromatogram supplied with testosterone enantate for peak identification CRS and the chromatogram obtained with reference solution (c) to identify the peak due to impurity E; use the chromatogram obtained with reference solution (d) to identify the peaks due to impurities B and D.
Relative retention With reference to testosterone enantate (retention time = about 22 min): impurity D = about 0.1; impurity B = about 0.7; impurity E = about 0.8; impurity F = about 0.85; impurity G = about 0.9.
System suitability Reference solution (a):
— resolution: minimum 1.3 between the peaks due to impurities F and G.
Limits:
— correction factor: for the calculation of content, multiply the peak area of impurity F by 6.3;
— impurity D: not more than 4 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.4 per cent);
— impurities E, F: for each impurity, not more than 3 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.3 per cent);
— impurity B: not more than twice the area of the principal peak in the chromatogram obtained with reference solution (b) (0.2 per cent);
— impurity G: not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.15 per cent);
— unspecified impurities: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (0.10 per cent);
— total: not more than 7 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.7 per cent);
— disregard limit: 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 per cent).
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in vacuo at a pressure not exceeding 0.7 kPa.
ASSAY
Dissolve 50.0 mg in anhydrous ethanol R and dilute to 100.0 mL with the same solvent. Dilute 2.0 mL of the solution to 100.0 mL with anhydrous ethanol R. Measure the absorbance (2.2.25) at the absorption maximum at 241 nm.
Calculate the content of C26H40O3 taking the specific absorbance to be 422.
STORAGE
Protected from light, at a temperature of 2 °C to 8 °C.
IMPURITIES
Specified impurities A, B, D, E, F, G, H.
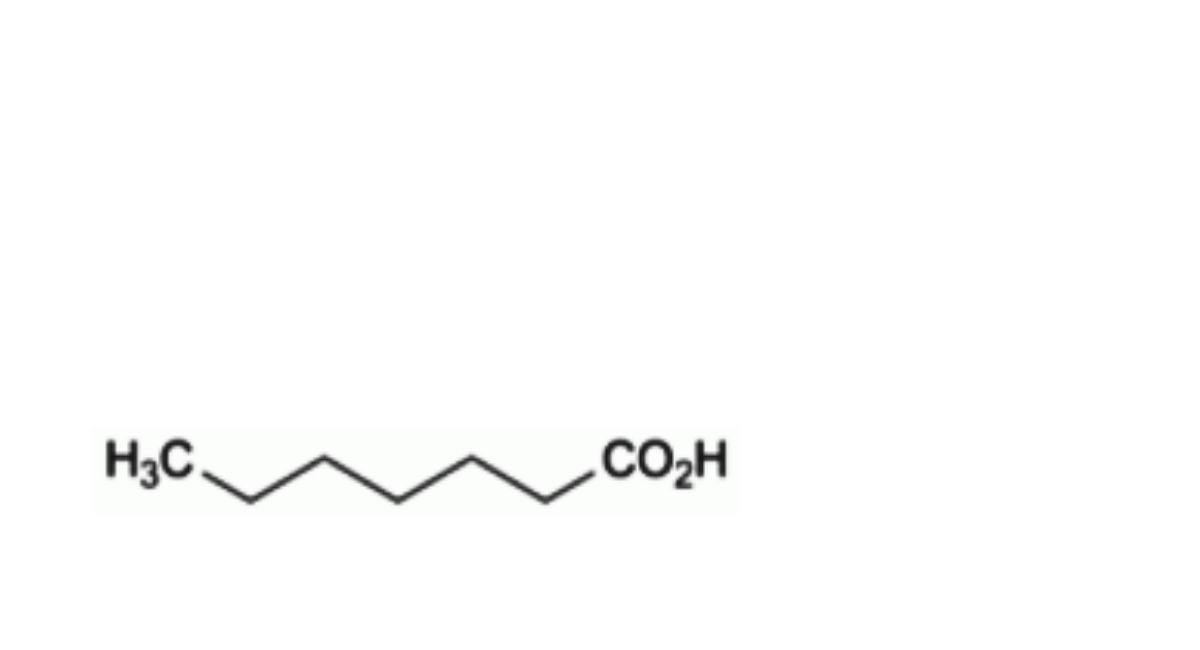
A. heptanoic acid,
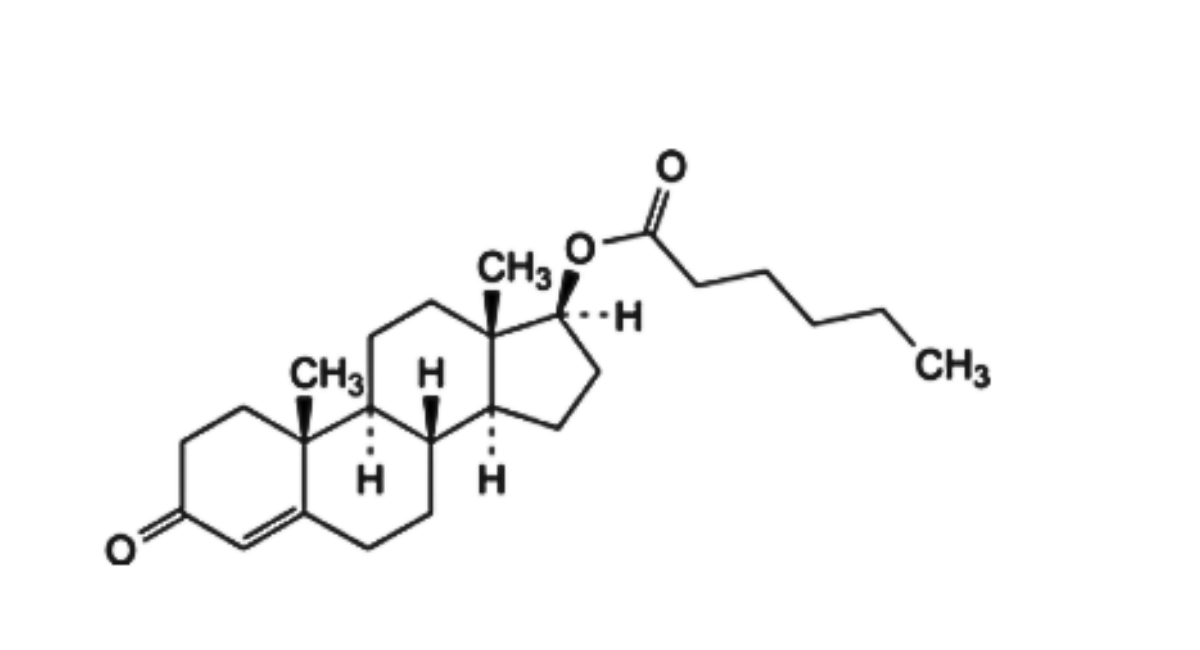
B. 3-oxoandrost-4-en-17β-yl hexanoate (testosterone caproate),
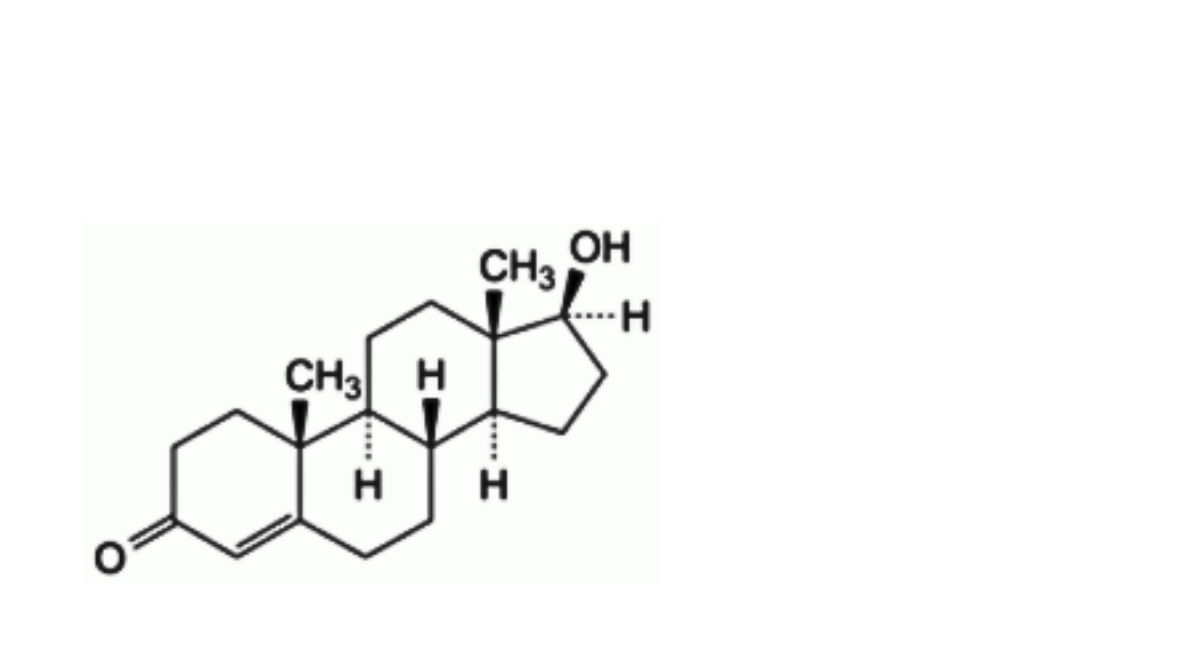
D. 17β-hydroxyandrost-4-en-3-one (testosterone),
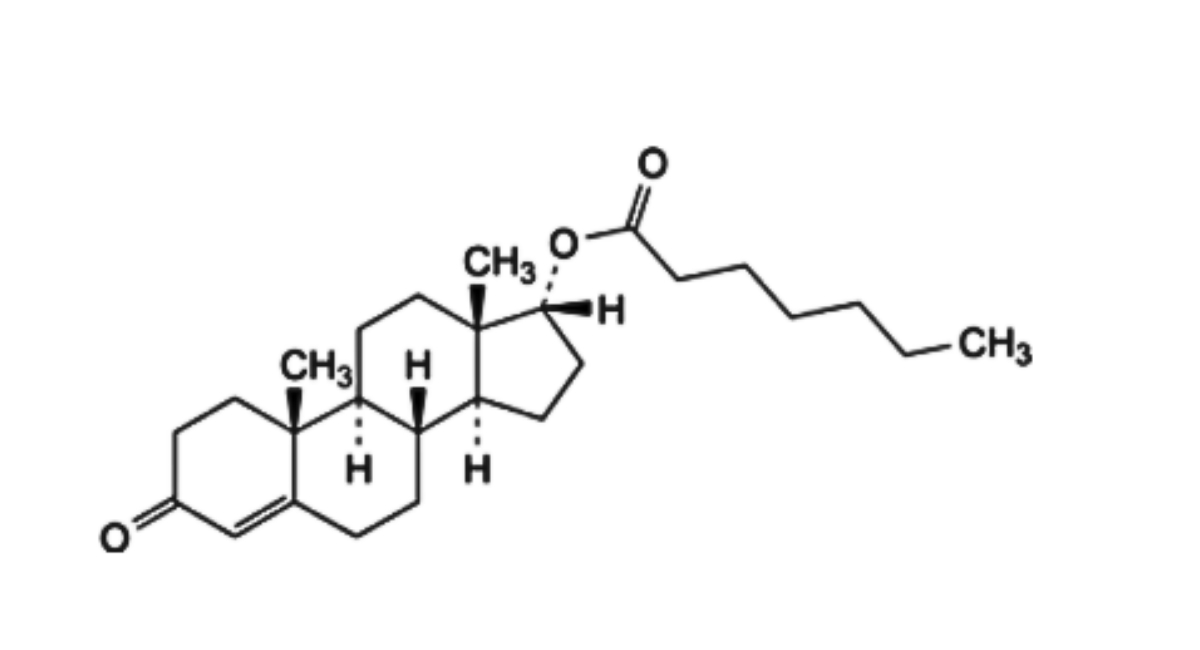
E. 3-oxoandrost-4-en-17α-yl heptanoate (17α-testosterone enantate),
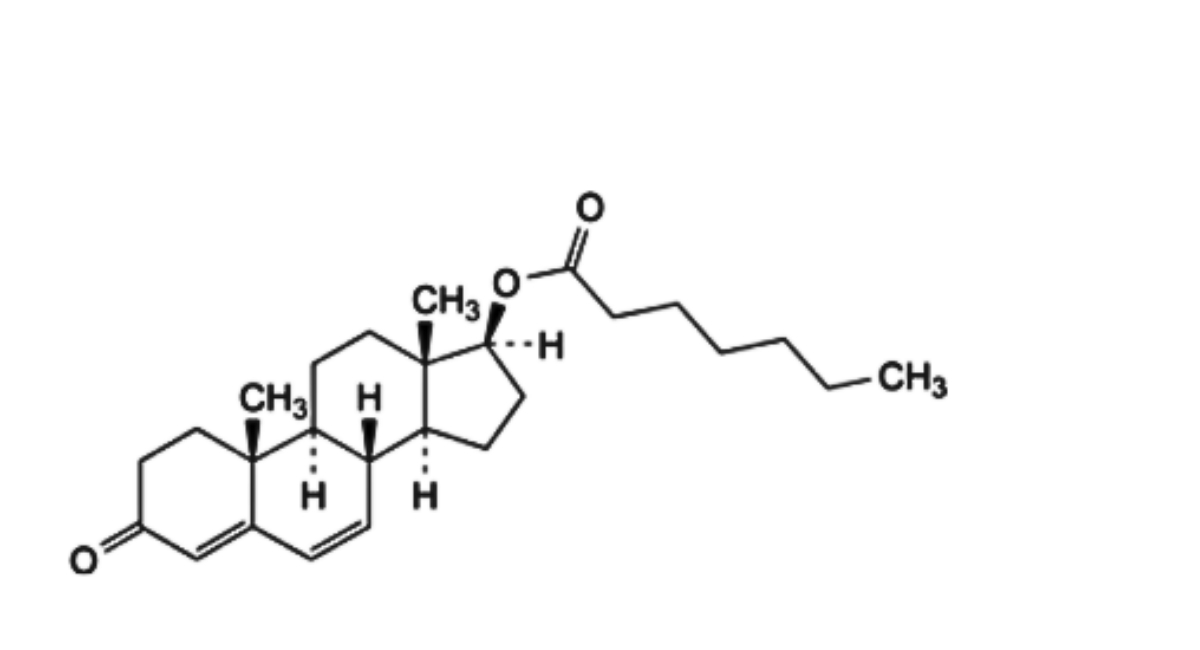
F. 3-oxoandrosta-4,6-dien-17β-yl heptanoate (Δ6-testosterone enantate),
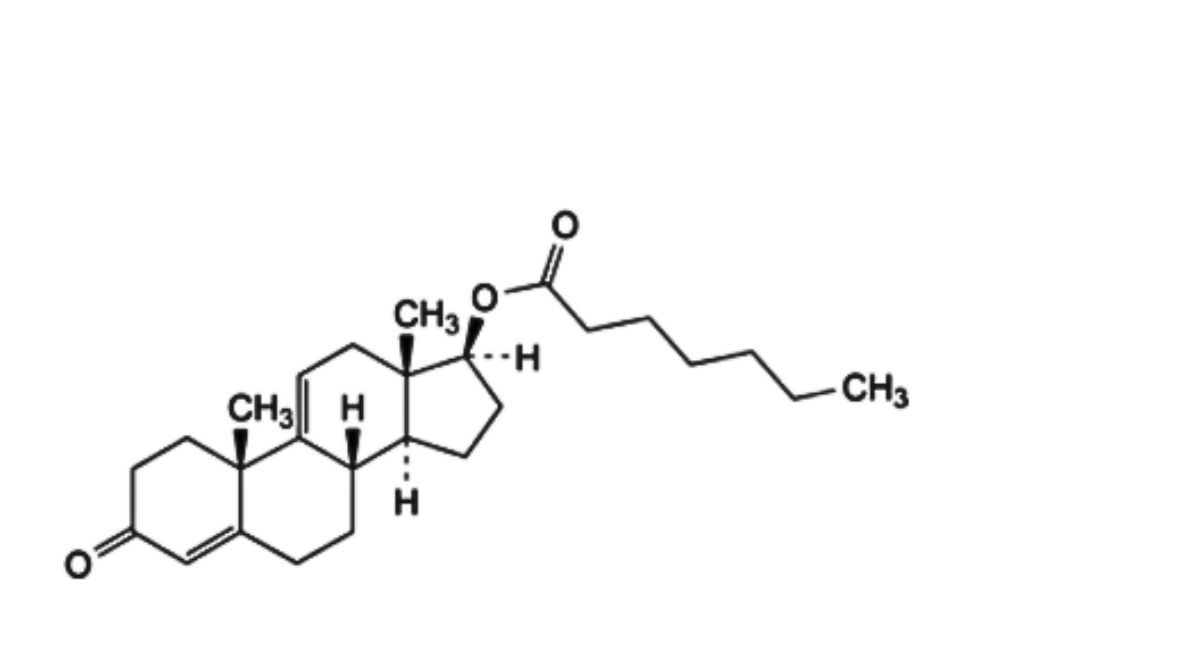
G. 3-oxoandrosta-4,9(11)-dien-17β-yl heptanoate (Δ9(11)-testosterone enantate),
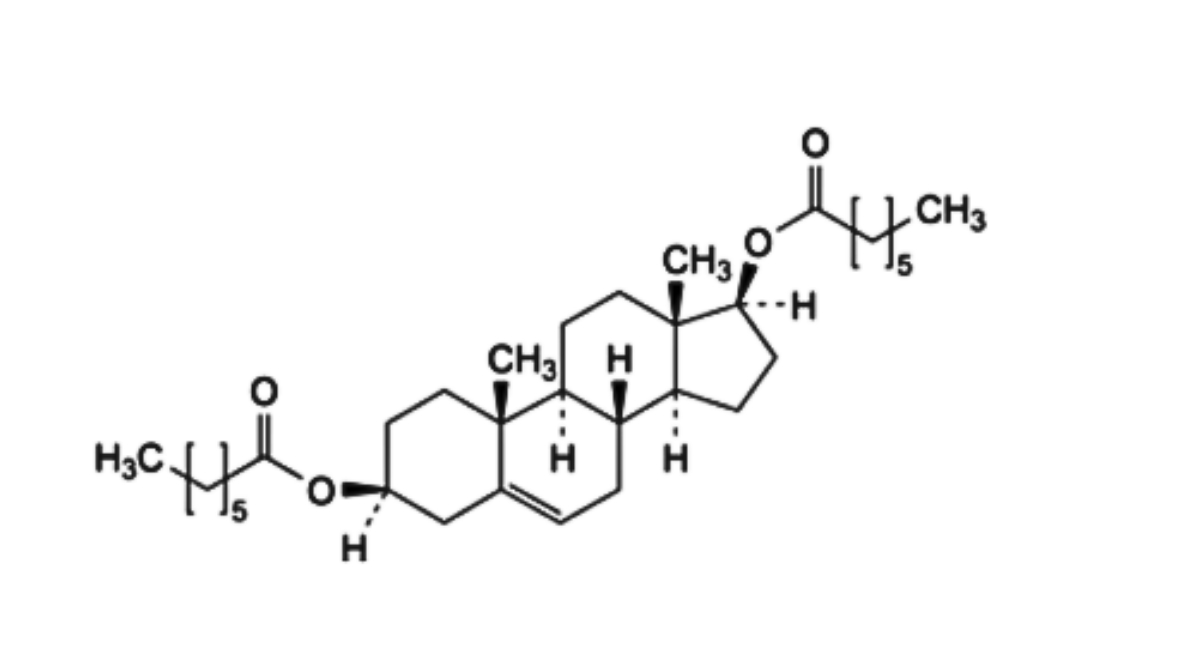
H. androst-5-ene-3β,17β-diyl diheptanoate.