(Ph. Eur. 11.6 update)
General Notices
(Ph. Eur. monograph 0954)
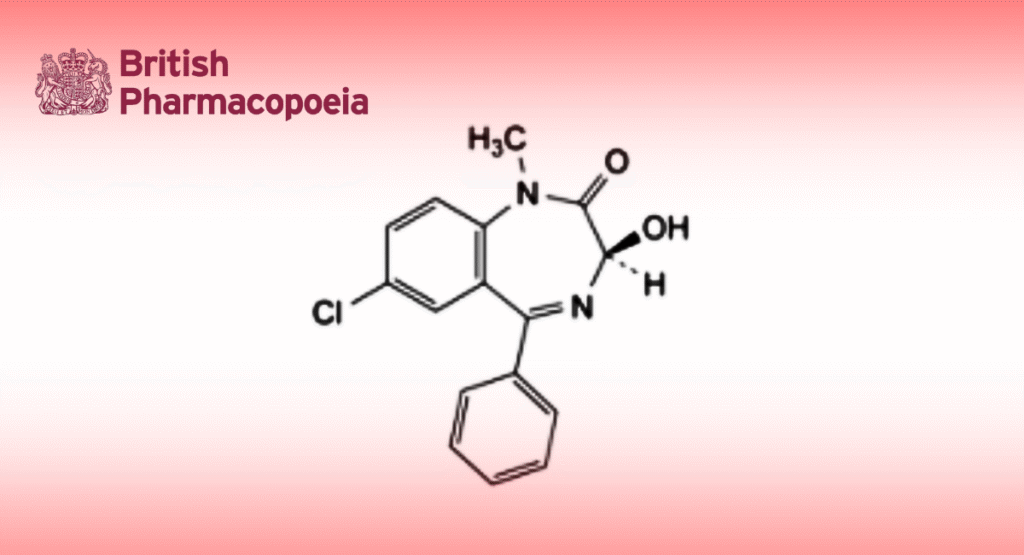
C16H13ClN2O2 300.7 846-50-4
Action and use
Benzodiazepine.
Preparations
Temazepam Oral Solution
Temazepam Tablets
Ph Eur
DEFINITION
(3RS)-7-Chloro-3-hydroxy-1-methyl-5-phenyl-1,3-dihydro-2H-1,4-benzodiazepin-2-one.
Content
98.0 per cent to 102.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Practically insoluble in water, freely soluble in methylene chloride, sparingly soluble in ethanol (96 per cent).
IDENTIFICATION
Infrared absorption spectrophotometry (2.2.24).
Comparison: temazepam for ID and assay CRS.
TESTS
Related substance
Liquid chromatography (2.2.29).
Solvent mixture: acetonitrile R, mobile phase A (30:70 V/V).
Test solution (a): Dissolve 20.0 mg of the substance to be examined in 6 mL of acetonitrile R and dilute to 20.0 mL with a 3.9 g/L solution of ammonium acetate R.
Test solution (b): Dilute 1.0 mL of test solution (a) to 10.0 mL with the solvent mixture.
Reference solution (a): Dissolve the contents of a vial of temazepam for system suitability CRS (containing impurities A, B, E and G) in 300 μL of acetonitrile R; then add 700 μL of mobile phase A.
Reference solution (b): Dilute 1.0 mL of test solution (a) to 100.0 mL with the solvent mixture. Dilute 1.0 mL of this solution to 10.0 mL with the solvent mixture.
Reference solution (c): Dissolve 20.0 mg of temazepam for ID and assay CRS in 6 mL of acetonitrile R and dilute to 20.0 mL with a 3.9 g/L solution of ammonium acetate R. Dilute 1.0 mL of the solution to 10.0 mL with the solvent mixture.
Column:
— size: l = 0.075 m, Ø = 4.6 mm;
— stationary phase: end-capped solid core octadecylsilyl silica gel for chromatography R (2.6 μm).
Mobile phase:
— mobile phase A: 3.9 g/L solution of ammonium acetate R;
— mobile phase B: acetonitrile R.
| Time (min) | Mobile phase A (per cent V/V) | Mobile phase B (per cent V/V) |
|---|---|---|
| 0 – 13 | 70 | 30 |
| 13 – 25 | 70 → 30 | 30 → 70 |
| 25 – 28 | 30 | 70 |
Flow rate: 1.0 mL/min.
Detection: Spectrophotometer at 254 nm.
Injection: 20 μL of test solution (a) and reference solutions (a) and (b).
Identification of impurities: Use the chromatogram supplied with temazepam for system suitability CRS and the chromatogram obtained with reference solution (a) to identify the peaks due to impurities A, B, E and G.
Relative retention:
With reference to temazepam (retention time = about 7 min):
— impurity B = about 0.57;
— impurity E = about 0.62;
— impurity G = about 1.2;
— impurity A = about 3.
System suitability: Reference solution (a):
— peak-to-valley ratio: minimum 1.5, where Hp = height above the baseline of the peak due to impurity G and Hv = height above the baseline of the lowest point of the curve separating this peak from the peak due to temazepam;
— resolution: minimum 2.0 between the peaks due to impurities B and E.
Calculation of percentage contents:
— correction factor: for the calculation of content, multiply the peak area of impurity E by 0.7;
— for each impurity, use the concentration of temazepam in reference solution (b).
Limits:
— impurity E: maximum 0.2 per cent;
— impurity A: maximum 0.05 per cent;
— unspecified impurities: for each impurity, maximum 0.10 per cent;
— total: maximum 0.5 per cent;
— reporting threshold: 0.05 per cent; do not disregard the peak due to impurity A;
— reporting threshold for impurity A: 0.025 per cent.
Loss on drying (2.2.32):
Maximum 0.5 per cent, determined on 1.000 g by drying in an oven at 105 °C for 4 h.
Sulfated ash (2.4.14):
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modification.
Injection: Test solution (b) and reference solution (c).
Calculate the percentage content of C16H13ClN2O2 taking into account the assigned content of temazepam for ID and assay CRS.
STORAGE
Protected from light.
IMPURITIES
Specified impurities: A, E.
Other detectable impurities: (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance.)
B, C, D, F, G.
methanone,](https://nhathuocngocanh.com/bp/wp-content/uploads/2025/11/A.-5-chloro-2-methylaminophenylphenylmethanone.png)
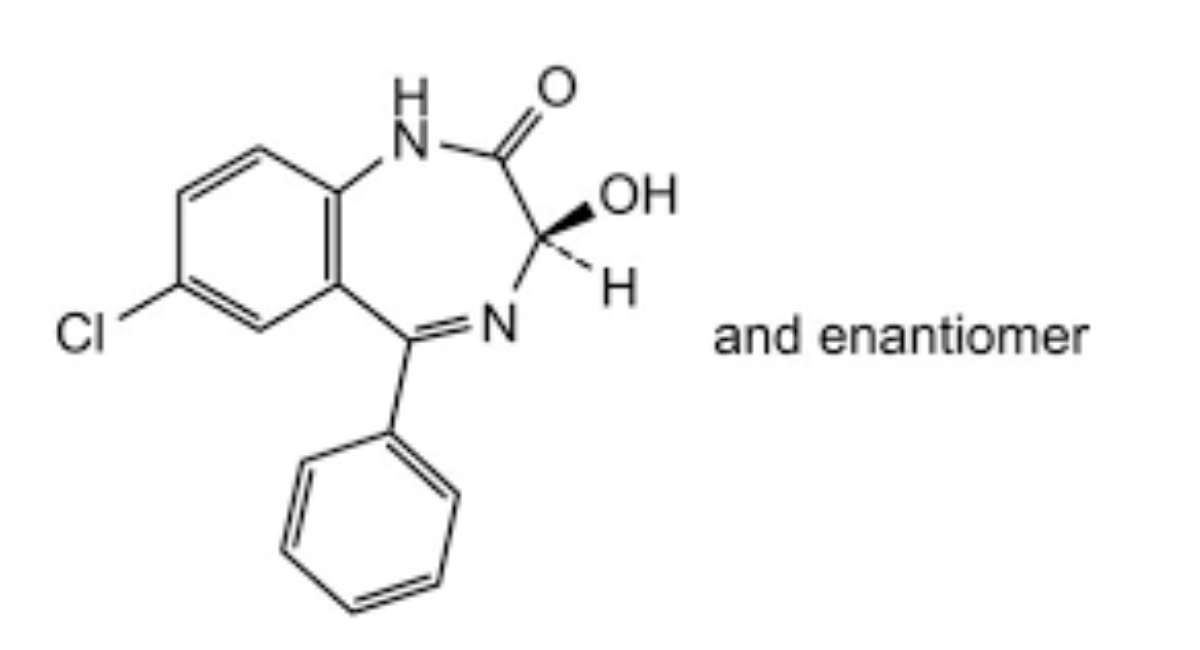
A. [5-chloro-2-(methylamino)phenyl](phenyl)methanone,
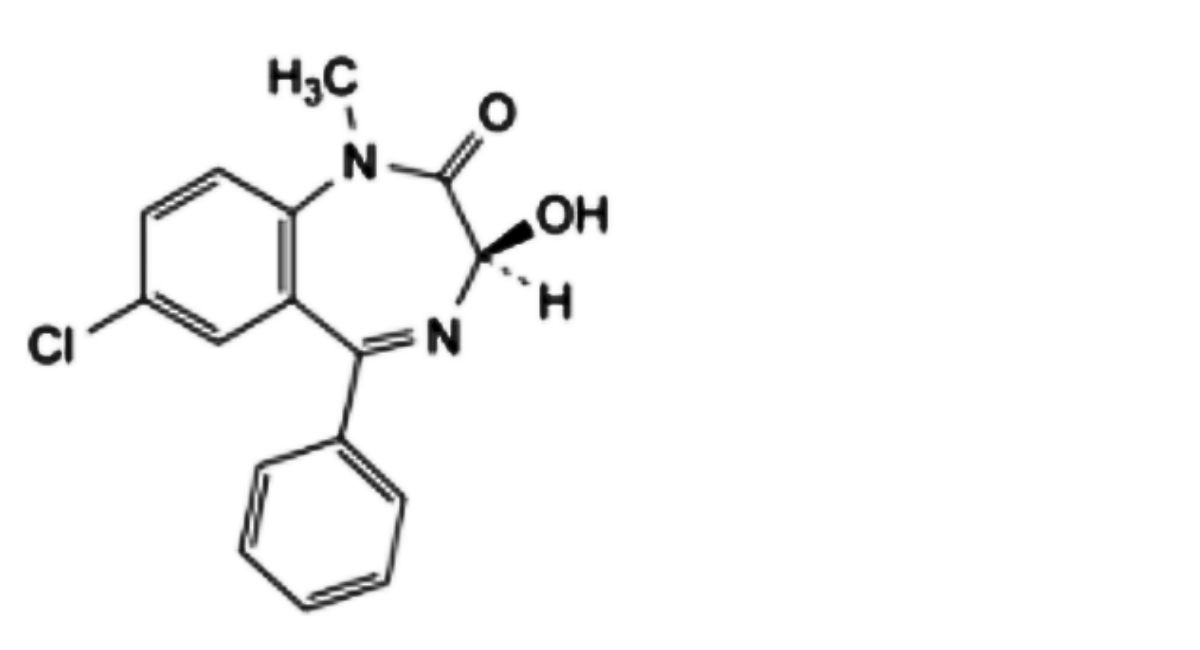
B. (3RS)-7-chloro-3-hydroxy-5-phenyl-1,3-dihydro-2H-1,4-benzodiazepin-2-one (oxazepam),
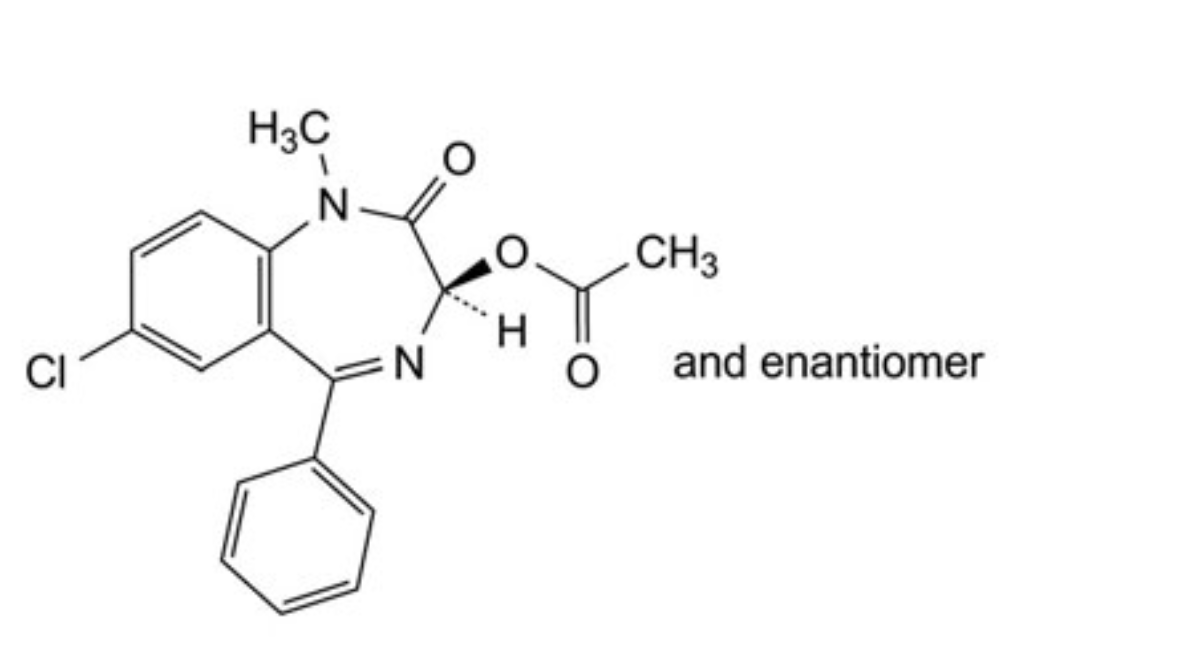
C. (3RS)-7-chloro-1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-1,4-benzodiazepin-3-yl acetate,
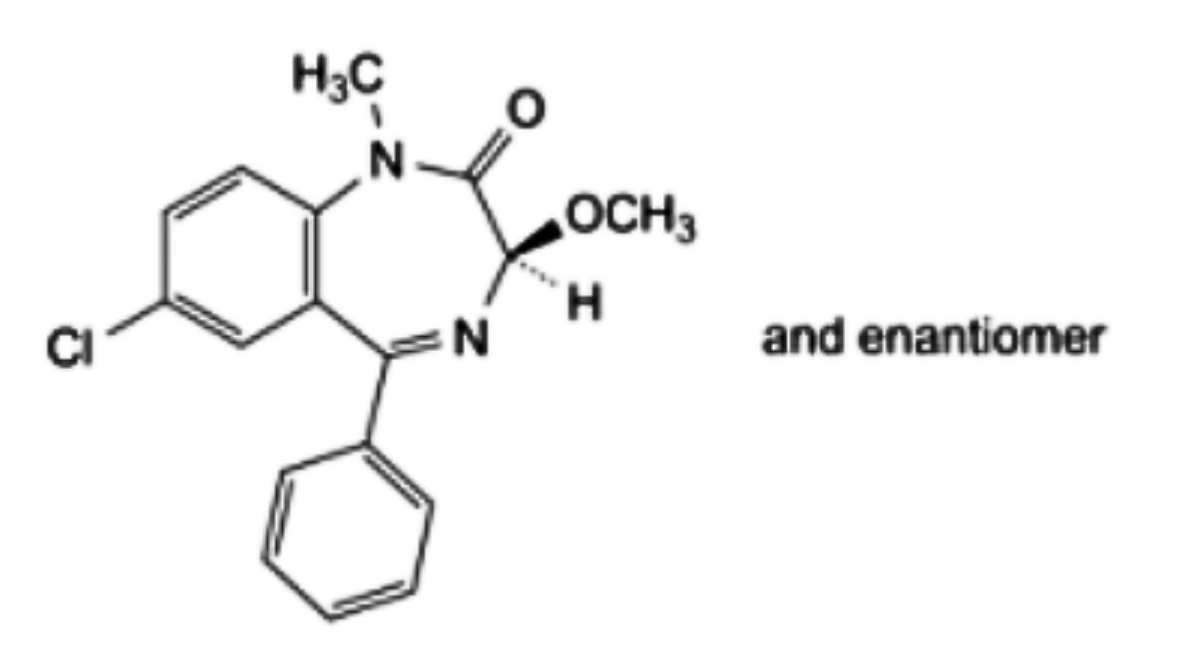
D. (3RS)-7-chloro-3-methoxy-1-methyl-5-phenyl-1,3-dihydro-2H-1,4-benzodiazepin-2-one,
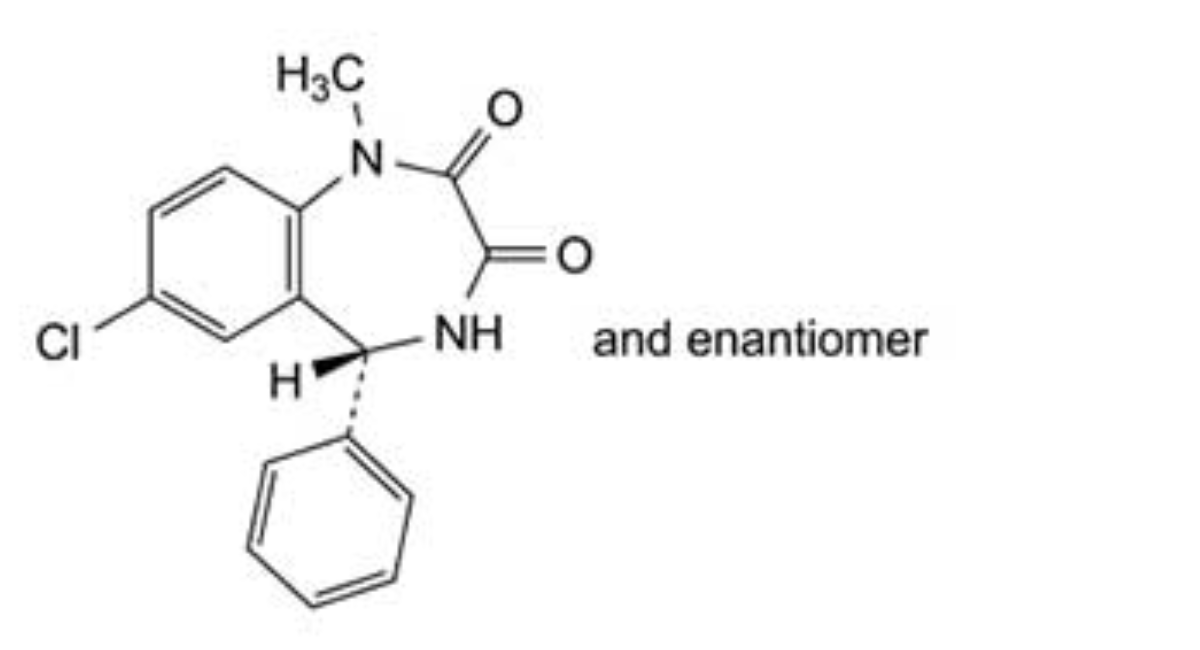
F. (5RS)-7-chloro-1-methyl-5-phenyl-4,5-dihydro-1H-1,4-benzodiazepine-2,3-dione,
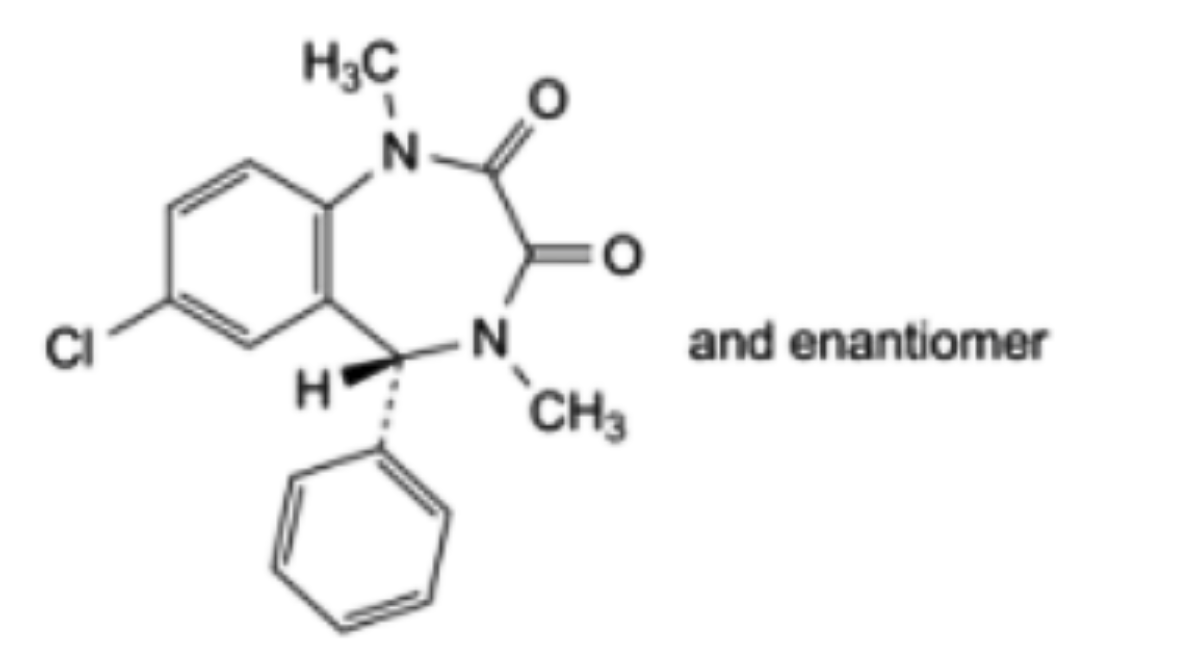
G. (5RS)-7-chloro-1,4-dimethyl-5-phenyl-4,5-dihydro-1H-1,4-benzodiazepine-2,3-dione,
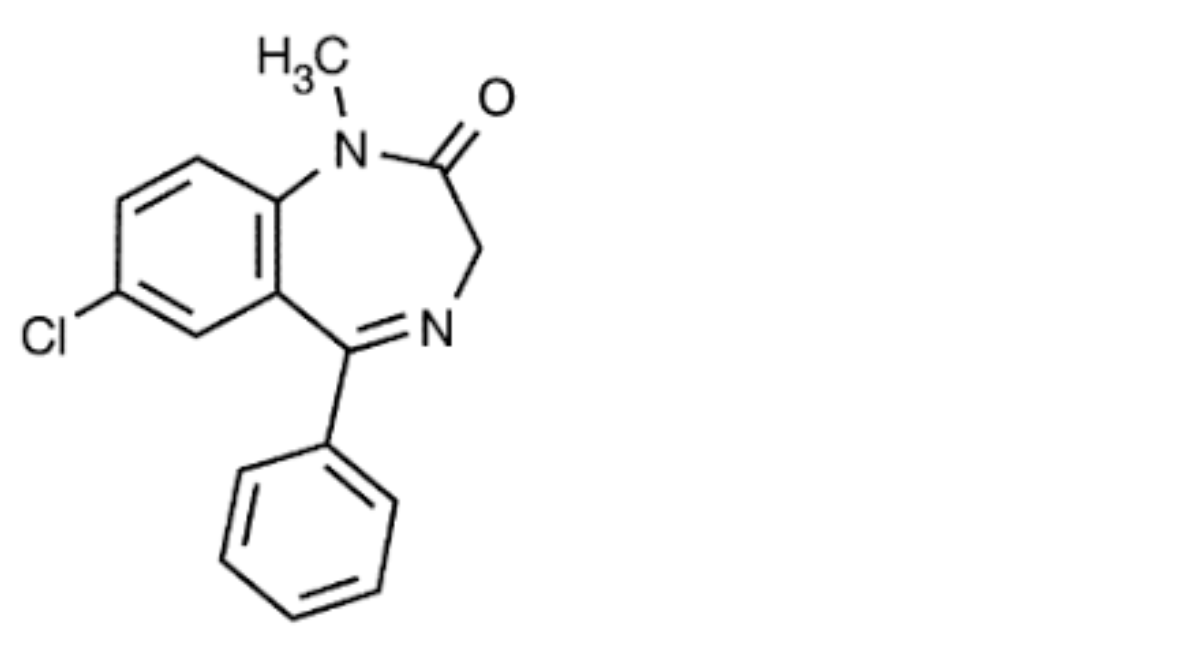
H. 7-chloro-1-methyl-5-phenyl-1,3-dihydro-2H-1,4-benzodiazepin-2-one (diazepam)