Edition: BP 2025 (Ph. Eur. 11.6 update)
General Notices
(Ph. Eur. monograph 2358)
Action and use
Glycopeptide antibacterial.
Preparation
Teicoplanin for Injection
Ph Eur
DEFINITION
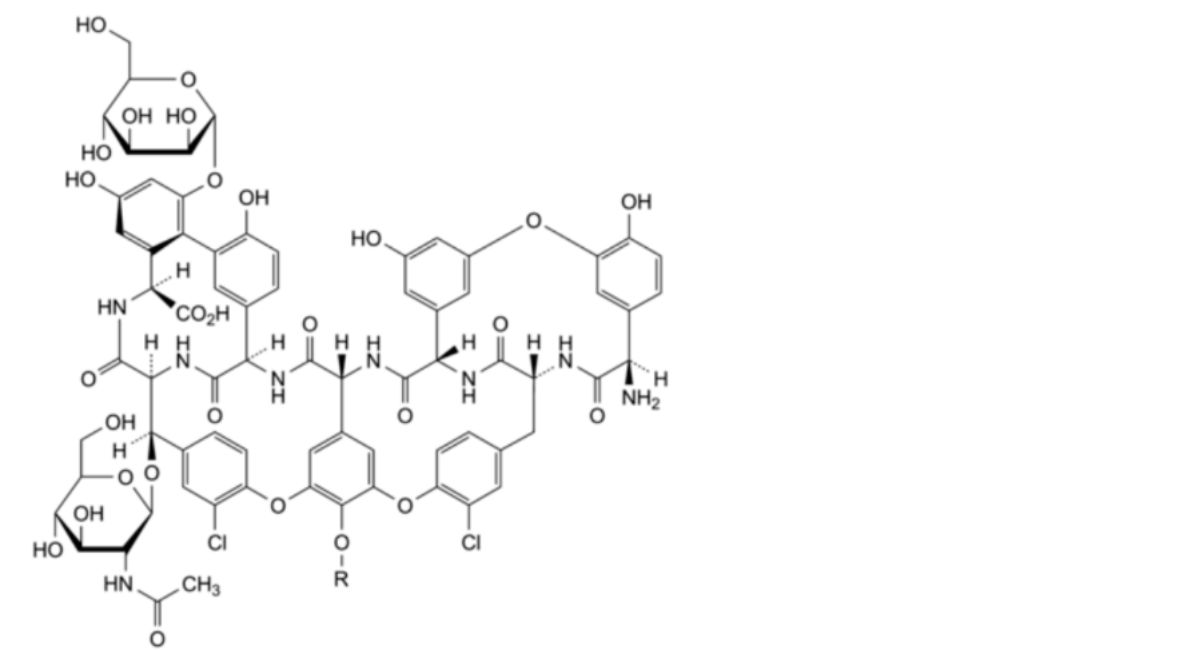
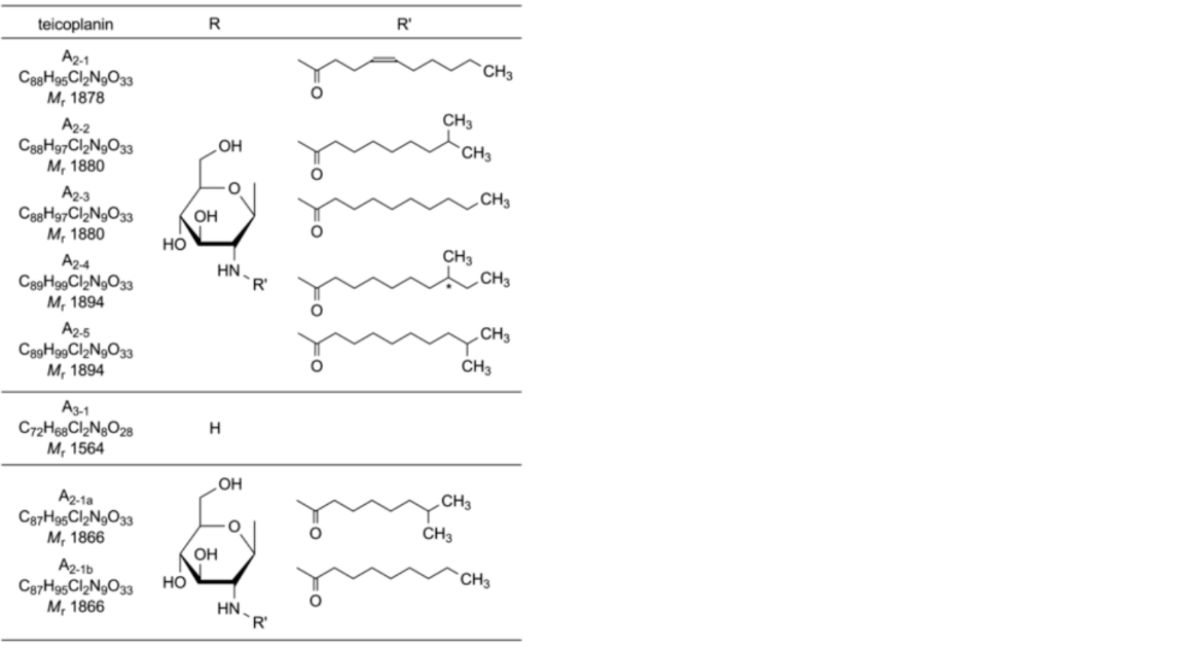
Mixture of glycopeptides produced by certain strains of Actinoplanes teichomyceticus sp.; the 6 principal components of the mixture are teicoplanins A2-1 to A2-5 and teicoplanin A3-1, and 2 minor components are teicoplanins A2-1a and A2-1b.
Fermentation product.
Potency: Minimum 900 IU/mg (anhydrous and sodium chloride-free substance).
CHARACTERS
Appearance
White or yellowish, amorphous powder.
Solubility
Freely soluble in water, sparingly soluble in dimethylformamide, practically insoluble in ethanol (96 per cent).
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24).
Comparison: teicoplanin for identification CRS.
B. Examine the chromatograms obtained in the test for composition.
Results: The principal peaks (teicoplanins A3-1, A2-1, A2-2, A2-3, A2-4 and A2-5) and the 2 minor peaks (A2-1a and A2-1b) in the chromatogram obtained with the test solution are similar in retention time and size to the principal peaks in the chromatogram obtained with reference solution (a).
TESTS
Appearance of solution:
The solution is clear (2.2.1) and not more intensely coloured than reference solution BY3 or B4 (2.2.2, Method I).
Dissolve 0.8 g in 10 mL of water R.
pH (2.2.3): 6.5 to 7.5.
Dissolve 0.50 g in carbon dioxide-free water R and dilute to 10 mL with the same solvent.
Composition:
Liquid chromatography (2.2.29); use the normalisation procedure.
Test solution: Dissolve 0.100 g of the substance to be examined in water R and dilute to 50.0 mL with the same solvent.
Reference solution (a): Dissolve 20 mg of teicoplanin for identification CRS in water R and dilute to 10.0 mL with the same solvent.
Reference solution (b): Dilute 1.0 mL of reference solution (a) to 10.0 mL with water R. Dilute 1.0 mL of this solution to 20.0 mL with water R.
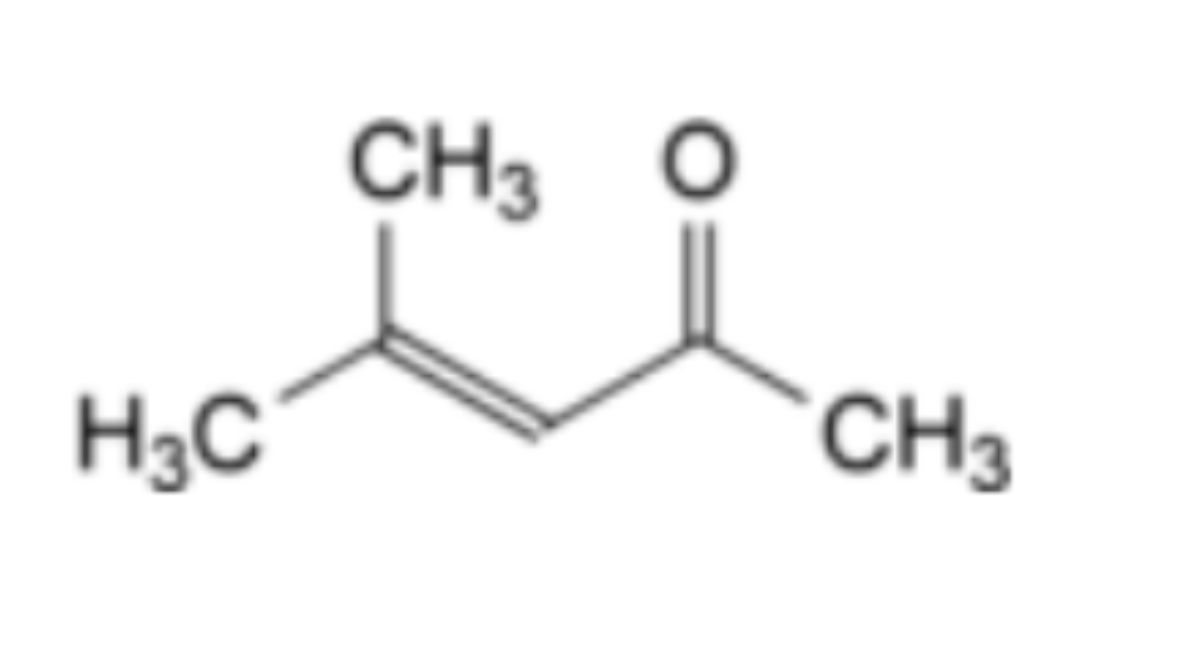
Reference solution (c): Dissolve 50.0 mg of mesityl oxide CRS (impurity A) in water R and dilute to 25.0 mL with the same solvent. Dilute 1.0 mL of this solution to 10.0 mL with water R. Dilute 1.0 mL of this solution to 100.0 mL with water R.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: spherical end-capped octadecylsilyl silica gel for chromatography R (5 μm).
Mobile phase:
— mobile phase A: mix 900 mL of a 3.0 g/L solution of anhydrous sodium dihydrogen phosphate R, adjusted to pH 6.0 with a 40 g/L solution of sodium hydroxide R, and 100 mL of acetonitrile R;
— mobile phase B: mix 300 mL of the same buffer and 700 mL of acetonitrile R.
| Time (min) | Mobile phase A (%) | Mobile phase B (%) |
| 0 – 30 | 100 → 50 | 0 → 50 |
| 30 – 31 | 50 → 10 | 50 → 90 |
| 31 – 35 | 10 | 90 |
Flow rate: 2.3 mL/min.
Detection: Spectrophotometer at 254 nm.
Injection: 20 μL of the test solution and reference solutions (a) and (b).
Identification:
Use the chromatogram supplied with teicoplanin for identification CRS and the chromatogram obtained with reference solution (a) to identify the groups and components.
Relative retention: With reference to teicoplanin A2-2 (retention time ≈ 18 min):
— Teicoplanin A3 group ≤ 0.70:
— Teicoplanin A3-1 = about 0.43.
— Teicoplanin A2 group > 0.70 including:
— Teicoplanin A2-1 group > 0.70 and < 1.00:
— Teicoplanin A2-1a = about 0.85;
— Teicoplanin A2-1b = about 0.88;
— Teicoplanin A2-1 = about 0.93.
— Teicoplanin A2-2 = 1.00.
— Teicoplanin A2-3 group > 1.00 and < 1.12:
— Teicoplanin A2-3 = about 1.03;
— Teicoplanin A2-4 = about 1.12.
— Teicoplanin A2-5 group > 1.12 and < 1.25:
— Teicoplanin A2-5 = about 1.15.
— Teicoplanin A2-6 group ≥ 1.25:
— Teicoplanin-like related substance RS A2-6a = about 1.25;
— Teicoplanin-like related substance RS A2-6b = about 1.30;
— Teicoplanin-like related substance RS A2-6c = about 1.38.
System suitability:
— The chromatogram obtained with reference solution (a) is similar to that supplied with teicoplanin for identification CRS.
— Resolution: minimum 1.0 between peaks due to A2-4 and A2-5 (reference a).
— Signal-to-noise ratio: minimum 40 for A2-2 (reference b).

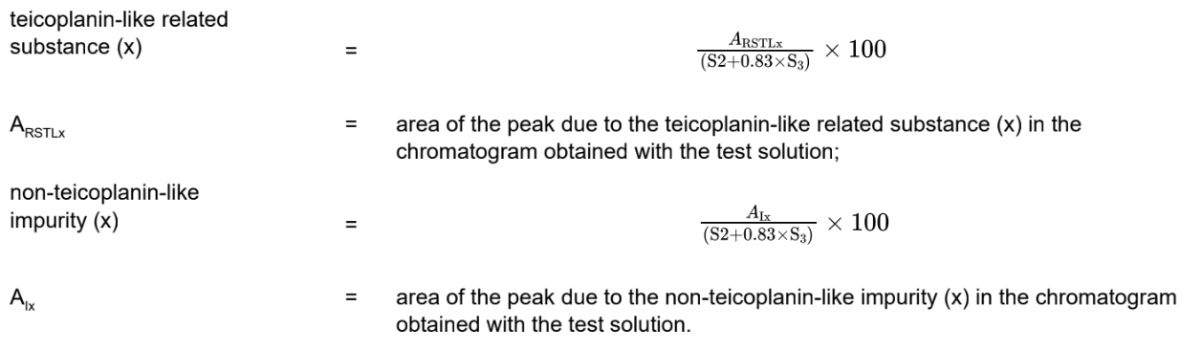
S2 = sum of the areas of the peaks due to teicoplanin A2 group in the chromatogram obtained with the test solution.
S3 = sum of the areas of the peaks due to teicoplanin A3 group in the chromatogram obtained with the test solution; disregard any peak due to impurity A.
S2-6 = sum of the areas of the peaks with a relative retention greater than or equal to 1.25 in the chromatogram obtained with the test solution.
S2-1 = sum of the areas of the peaks due to teicoplanin A2-1 group in the chromatogram obtained with the test solution.
A2-1a = area of the peak due to teicoplanin A2-1a in the chromatogram obtained with the test solution.
A2-1b = area of the peak due to teicoplanin A2-1b in the chromatogram obtained with the test solution.
A2-1 = area of the peak due to teicoplanin A2-1 in the chromatogram obtained with the test solution.
A2-2 = area of the peak due to teicoplanin A2-2 in the chromatogram obtained with the test solution.
S2-3 = sum of the areas of the peaks due to teicoplanin A2-3 group in the chromatogram obtained with the test solution.
A2-3 = area of the peak due to teicoplanin A2-3 in the chromatogram obtained with the test solution.
A2-4 = area of the peak due to teicoplanin A2-4 in the chromatogram obtained with the test solution.
S2-5 = sum of the areas of the peaks due to teicoplanin A2-5 group in the chromatogram obtained with the test solution.
A2-5 = area of the peak due to teicoplanin A2-5 in the chromatogram obtained with the test solution.
Limits
— Teicoplanin A2 group: 84.0 % – 98.0 %.
— Teicoplanin A2-2: 37.0 % – 50.0 %.
— Teicoplanin A2-1 group: 10.0 % – 19.0 %.
— Teicoplanin A2-5 group: 7.0 % – 17.0 %.
— Teicoplanin A2-4: 7.0 % – 15.0 %.
— Teicoplanin A2-5: 7.0 % – 15.0 %.
— Teicoplanin A2-3 group: 5.0 % – 11.0 %.
— Teicoplanin A3 group: 4.0 % – 12.0 %.
— Teicoplanin A2-3: 4.0 % – 8.5 %.
— Teicoplanin A2-1: 2.0 % – 7.0 %.
— Teicoplanin A2-1a: 0.5 % – 5.5 %.
— Teicoplanin A2-1b: 0.5 % – 4.0 %.
— Teicoplanin A2-6 group: ≤ 5.0 %.
— Disregard limit: 0.25 %.
Related substances:
Liquid chromatography (2.2.29) as described in the test for composition (normalisation procedure).
Use the chromatogram obtained with reference solution (a) to identify all peaks present above the disregard limit as teicoplanin-like related substances. Any peak present in any part of the chromatogram obtained with the test solution that cannot be correlated to a peak above the disregard limit in reference solution (a) should be considered as a non-teicoplanin-like impurity, unless it is characterized by other means.
A teicoplanin-like related substance is defined as a substance that shares the same glycopeptide core structure of the parent molecule, composed of a linear heptapeptide aglycone, an α-D-mannose and an acetyl-β-D-glucosamine. The R′ side chains in the teicoplanin-like related substances RS A2-6a, RS A2-6b and RS A2-6c are unknown.
Calculate the percentage contents using the following equations:
Limits:
— RS A2-6c: ≤ 2.5 %.
— RS A2-6a: ≤ 1.5 %.
— RS A2-6b: ≤ 1.5 %.
— Any non-teicoplanin-like impurity (other than A): ≤ 0.5 %.
— Total non-teicoplanin-like impurities (other than A): ≤ 1.5 %.
Impurity A:
Liquid chromatography (2.2.29) as described in the test for composition, with the following modifications.
Injection: 20 μL of the test solution and reference solution (c).
Relative retention: With reference to A2-2 (retention ≈ 18 min): impurity A ≈ 0.6.
Calculation of content: Use the concentration of impurity A in reference solution (c).
Limit:
— Impurity A ≤ 0.2 %.
Chlorides:
Maximum 5.0 %, expressed as sodium chloride (anhydrous substance).
Dissolve 1.000 g in 300 mL of water R, stir and acidify with 2 mL of nitric acid R.
Titrate with 0.1 M silver nitrate, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M AgNO₃ ≡ 5.844 mg NaCl.
Water (2.5.12): Maximum 15.0 % (on 0.300 g).
ASSAY
Carry out the microbiological assay of antibiotics (2.7.2), using the diffusion method.
Use teicoplanin CRS as the reference substance.
STORAGE
Protected from light, at 2 °C to 8 °C.
IMPURITIES
Specified impurity: A.