BP 2025 (Ph. Eur. 11.6 update)
(Ph. Eur. monograph 2244)
Tacrolimus Monohydrate – British Pharmacopoeia 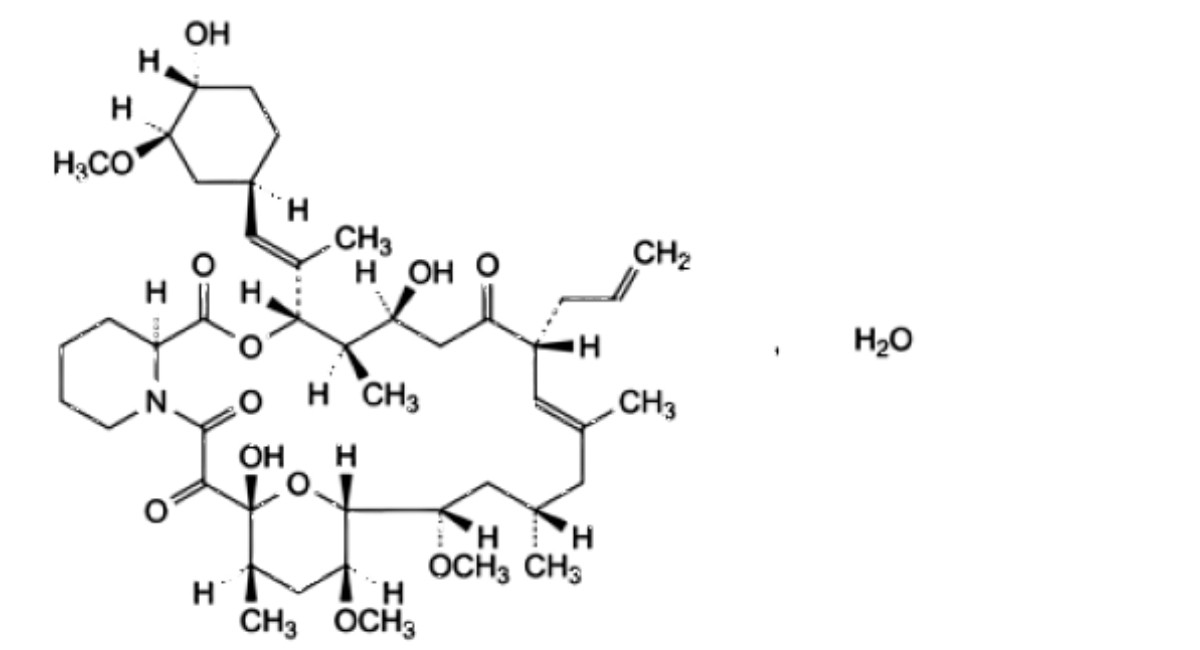
C44H69NO12,H2O 822 109581-93-3
Action and use
Calcineurin inhibitor; immunosuppressant.
Ph Eur
DEFINITION
(3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19R,26aS)-5,19-dihydroxy-3-[(1 E)-1-[(1 R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]prop-1-en-2-yl]-14,16-dimethoxy-4,10,12,18-tetramethyl-8-(prop-2-en-1-yl)-3,4,5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-octadecahydro-7H-15,19-epoxypyrido[2,1-c]oxaazacyclotricosine-1,7,20,21(23R)-tetrone monohydrate.
Substance produced by the growth of certain strains of Streptomyces tsukubaensis.
Content
97.0 per cent to 102.0 per cent for the sum of tacrolimus, tacrolimus compound I and tacrolimus compound II (anhydrous substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Practically insoluble in water, soluble in ethanol (96 per cent), practically insoluble in heptane.
IDENTIFICATION
Infrared absorption spectrophotometry (2.2.24).
Comparison tacrolimus monohydrate CRS.
TESTS
Appearance of solution
The solution is clear (2.2.1) and not more intensely coloured than reference solution GY7 (2.2.2, Method II).
Dissolve 0.20 g in ethanol (96 per cent) R and dilute to 20.0 mL with the same solvent.
Related substances
Liquid chromatography (2.2.29). Carry out the test protected from light. Allow the test and reference solutions to stand for 3 h at room temperature before use.
Solvent mixture water R, acetonitrile R (30:70 V/V).
Test solution Dissolve 30.0 mg of the substance to be examined in the solvent mixture and dilute to 10.0 mL with the solvent mixture.
Reference solution (a) Dissolve 30.0 mg of tacrolimus monohydrate CRS in the solvent mixture and dilute to 10.0 mL with the solvent mixture.
Reference solution (b) Dissolve 3.0 mg of tacrolimus for system suitability CRS (containing impurity A) in the solvent mixture and dilute to 1.0 mL with the solvent mixture.
Reference solution (c) Dilute 1.0 mL of the test solution to 100.0 mL with the solvent mixture. Dilute 1.0 mL of this solution to 10.0 mL with the solvent mixture.
Column:
- size: l = 0.15 m, Ø = 4.6 mm;
- stationary phase: end-capped octadecylsilyl silica gel for chromatography R (3 μm);
- temperature: 60 °C.
Mobile phase:
- mobile phase A: mix 20 volumes of a mixture of 19 volumes of 1,1-dimethylethyl methyl ether R and 81 volumes of acetonitrile for chromatography R with 80 volumes of a 0.04 per cent V/V solution of phosphoric acid R;
- mobile phase B: mix 20 volumes of a 0.04 per cent V/Vsolution of phosphoric acid R with 80 volumes of a mixture of 19 volumes of 1,1-dimethylethyl methyl ether R and 81 volumes of acetonitrile for chromatography R;
| Time (min) | Mobile phase A (per cent V/V) | Mobile phase B (per cent V/V) |
| 0 – 30 | 72 | 28 |
| 30 – 53 | 72 -> 15 | 28 -> 85 |
Flow rate 1.5 mL/min.
Detection Spectrophotometer at 220 nm.
Autosampler Set at 4 °C.
Injection 20 μL of the test solution and reference solutions (b) and (c).
Identification of impurities Use the chromatogram supplied with tacrolimus for system suitability CRS and the chromatogram obtained with reference solution (b) to identify the peak due to impurity A.
Relative retention With reference to tacrolimus (retention time = about 24 min): tacrolimus compound I = broad peak at about 0.5; impurity B = sharp peak at about 0.5; tacrolimus compound II = about 0.6; impurity A = about 0.9.
System suitability Reference solution (b):
- resolution: minimum 3.0 between the peaks due to impurity A and tacrolimus.
Integration The peak due to impurity B, which co-elutes with tacrolimus compound I, is a rider peak that is to be integrated using tangential skimming. It is necessary that the integration area of the peak due to tacrolimus compound I preserves the Gaussian peak shape.
Calculation of percentage contents:
- for each impurity, use the concentration of tacrolimus monohydrate in reference solution (c).
Limits:
- impurity A: maximum 0.5 per cent;
- unspecified impurities: for each impurity, maximum 0.15 per cent;
- total: maximum 1.0 per cent;
- reporting threshold: 0.05 per cent; disregard the peaks due to tacrolimus compound I and tacrolimus compound II.
Water (2.5.12)
1.5 per cent to 4.0 per cent, determined on 0.250 g.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modification.
Injection Test solution and reference solution (a).
Calculate the percentage content of the sum of tacrolimus, tacrolimus compound I and tacrolimus compound II taking into account the assigned content of tacrolimus monohydrate CRS.
STORAGE
In an airtight container. If the substance is sterile, the container is also sterile and tamper-evident.
IMPURITIES
Specified impurities A.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities. It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) B, C, D, E, H, I.
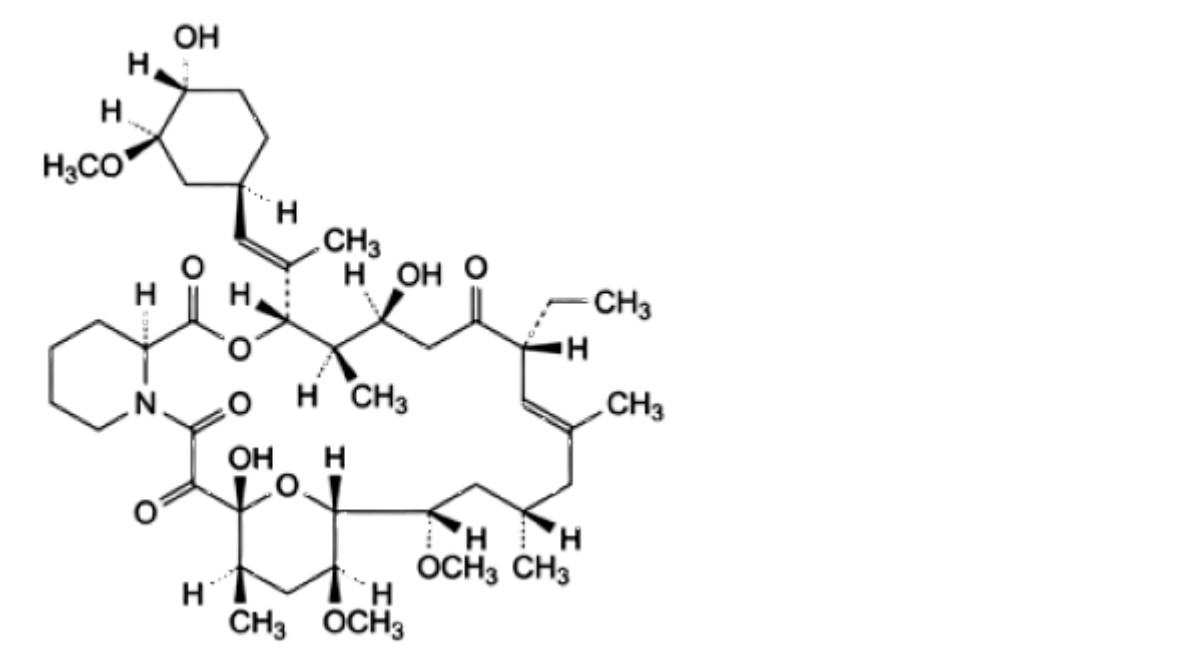
A. (3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19R,26aS)-8-ethyl-5,19-dihydroxy-3-[(1E)-1-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]prop-1-en-2-yl]-14,16-dimethoxy-4,10,12,18-tetramethyl-3,4,5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-octadecahydro-7R-15,19-epoxypyrido[2,1-c][1,4]oxaazacyclotricosine-1,7,20,21(23R)-tetrone,
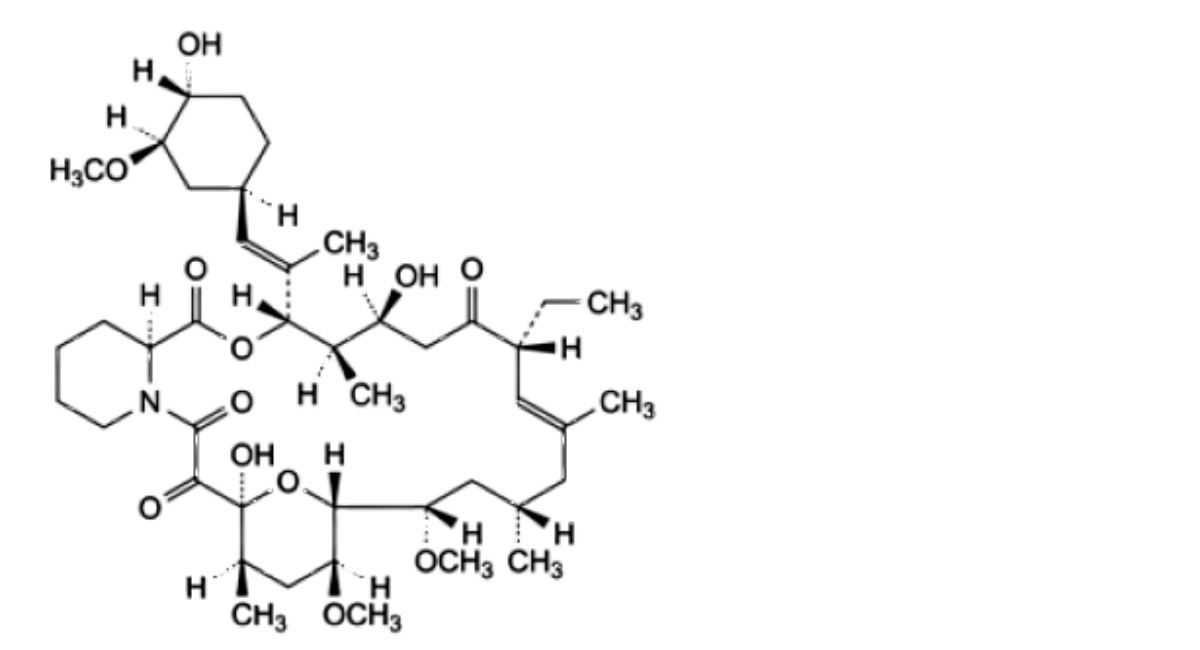
B. (3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19S,26aS)-8-ethyl-5,19-dihydroxy-3-[(1E)-1-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]prop-1-en-2-yl]-14,16-dimethoxy-4,10,12,18-tetramethyl-3,4,5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-octadecahydro-7R-15,19-epoxypyrido[2,1-c][1,4]oxaazacyclotricosine- 1,7,20,21(23R)-tetrone,
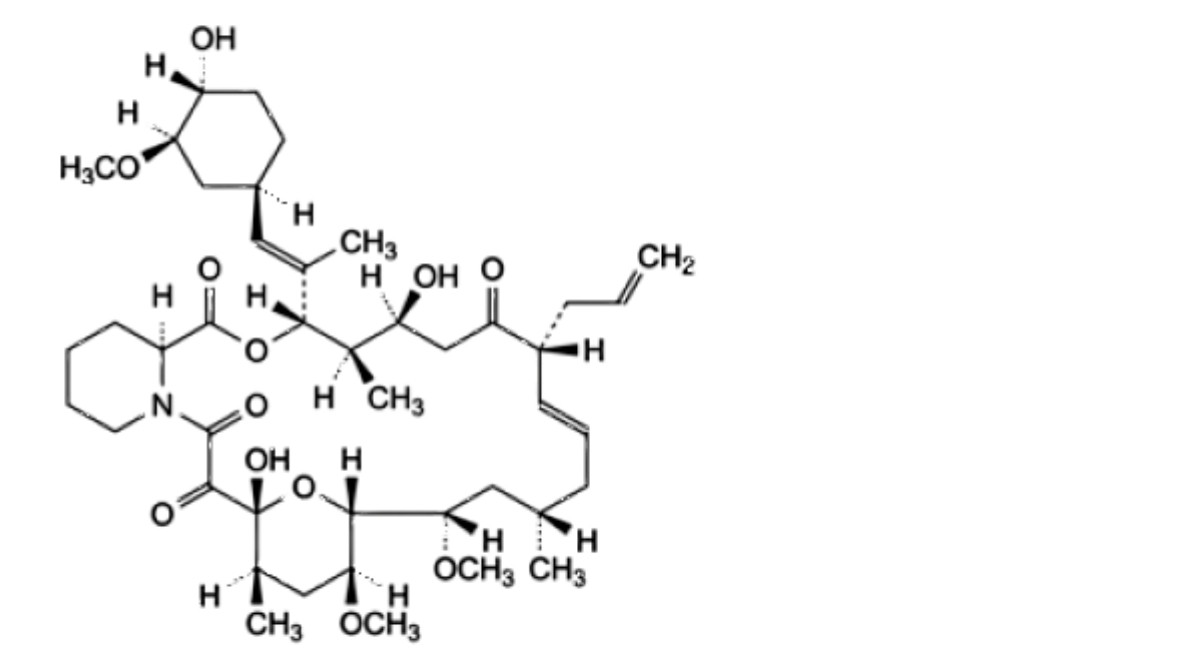
C. (3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19R,26aS)-5,19-dihydroxy-3-[(1 E)-1-[(1 R,3R,4R)-4-hydroxy-3- methoxycyclohexyl]prop-1-en-2-yl]-14,16-dimethoxy-4,12,18-trimethyl-8-(prop-2-en-1-yl)-3,4,5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-octadecahydro-7H-15,19-epoxypyrido[2,1-c]oxaazacyclotricosine-1,7,20,21(23R)-tetrone,
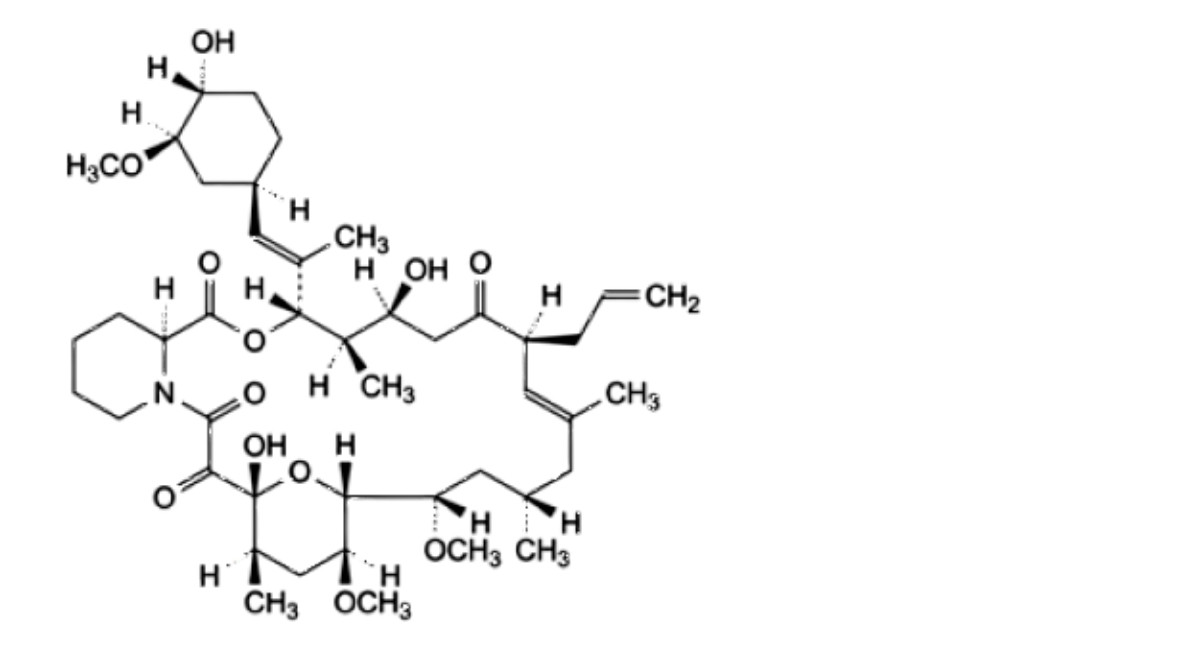
D. (3S,4R,5S,8S,9E,12S,14S,15R,16S,18R,19R,26aS)-5,19-dihydroxy-3-[(1E)-1-[(1R,3R,4R)-4-hydroxy-3- methoxycyclohexyl]prop-1-en-2-yl]-14,16-dimethoxy-4,10,12,18-tetramethyl-8-(prop-2-en-1-yl)-3,4,5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-octadecahydro-7H-15,19-epoxypyrido[2,1-c]oxaazacyclotricosine-1,7,20,21(23R)-tetrone,
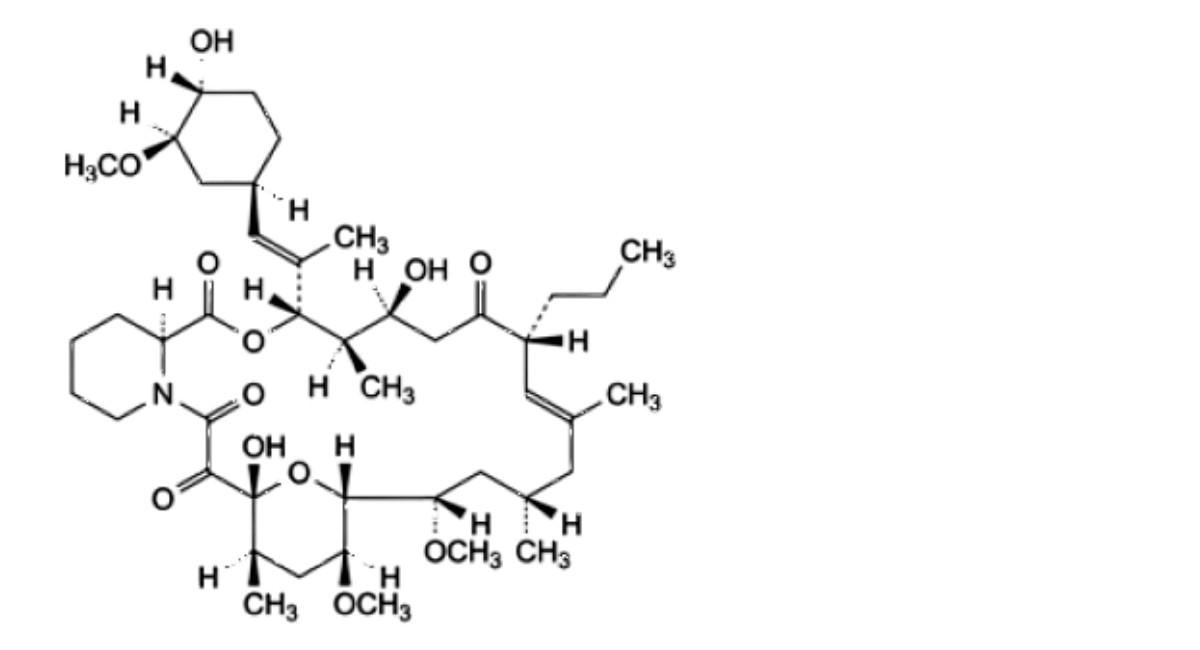
E. (3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19R,26aS)-5,19-dihydroxy-3-[(1E)-1-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]prop-1-en-2-yl]-14,16-dimethoxy-4,10,12,18-tetramethyl-8-propyl-3,4,5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-octadecahydro-7R-15,19-epoxypyrido[2,1-c][1,4]oxaazacyclotricosine- 1,7,20,21(23R)-tetrone,
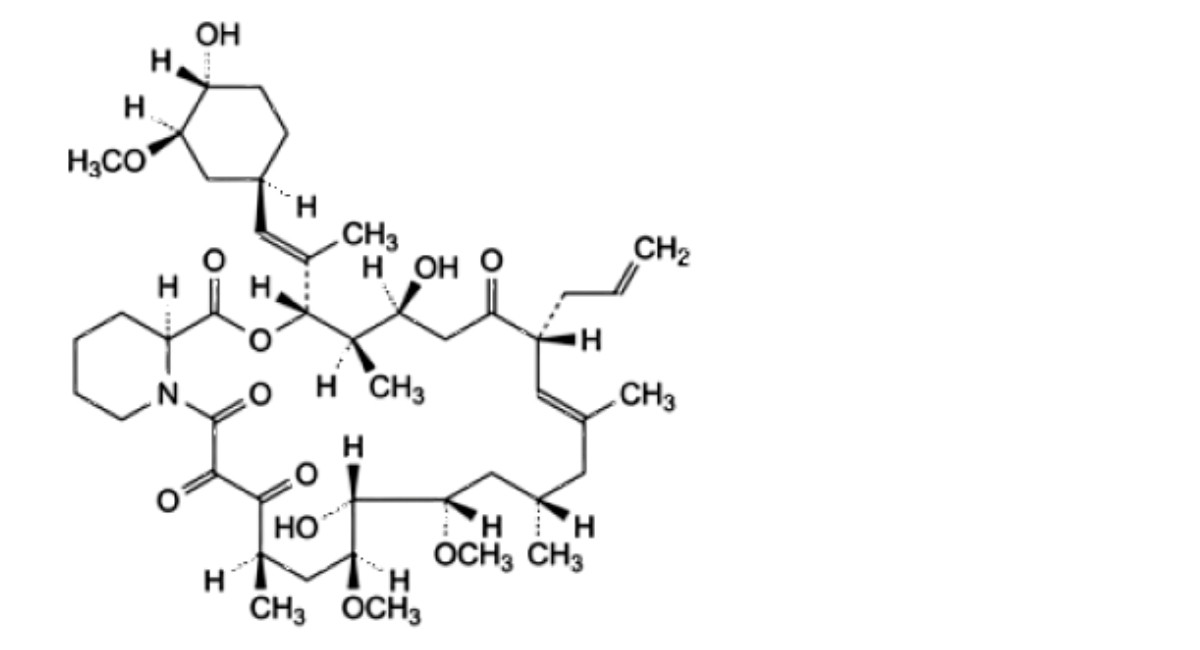
F. (3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19R,26aS)-5,15-dihydroxy-3-[(1 E)-1-[(1 R,3R,4R)-4-hydroxy-3- methoxycyclohexyl]prop-1-en-2-yl]-14,16-dimethoxy-4,10,12,18-tetramethyl-8-(prop-2-en-1- yl)-3,4,5,6,11,12,13,14,15,16,17,18,24,25,26,26a-hexadecahydro-7R-pyrido[2,1-c][1,4]oxaazacyclotricosine- 1,7,19,20,21(8R,23R)-pentone (tacrolimus compound I),
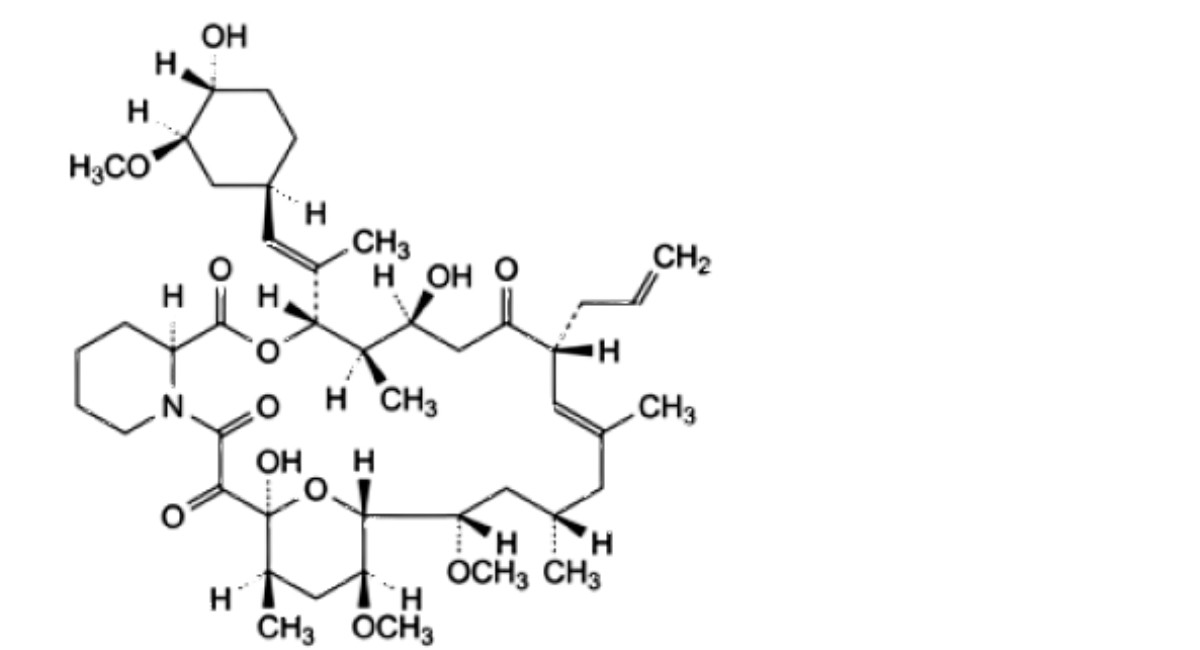
G. (3S,4R,5S,8R,9E,12S,14S,15R,16S,18R,19S,26aS)-5,19-dihydroxy-3-[(1E)-1-[(1R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]prop-1-en-2-yl]-14,16-dimethoxy-4,10,12,18-tetramethyl-8-(prop-2-en-1-yl)-3,4,5,6,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-octadecahydro-7H-15,19-epoxypyrido[2,1-c]oxaazacyclotricosine-1,7,20,21(23R)-tetrone (tacrolimus compound II),
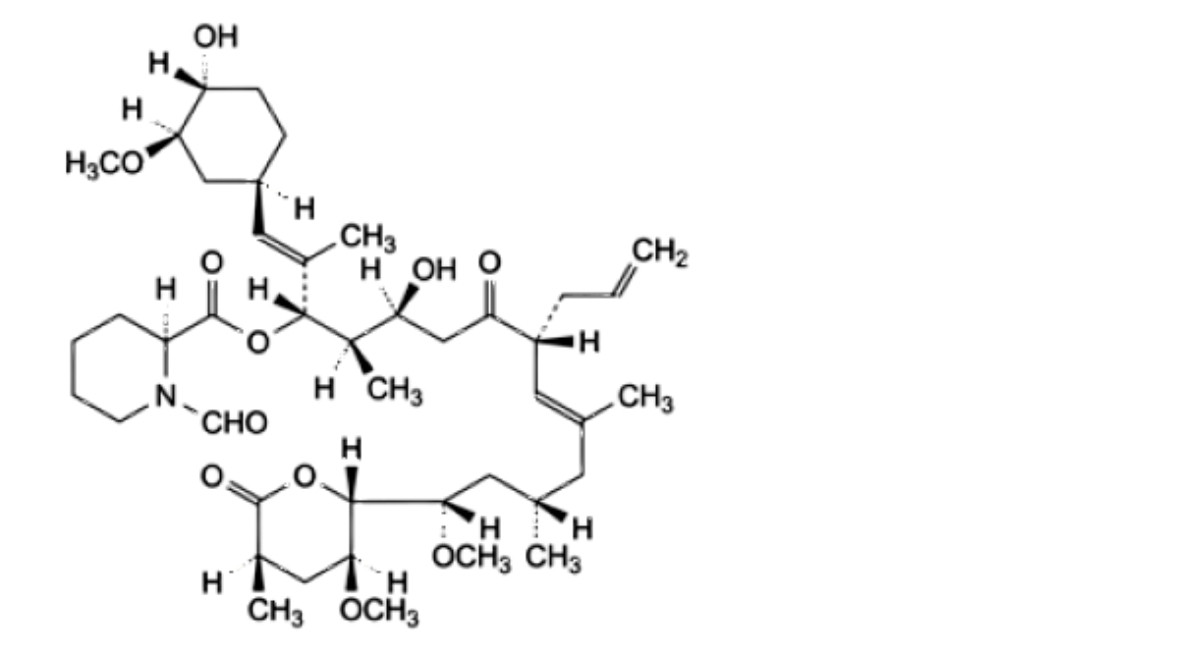
H. (1E,3S,4R,5S,8R,9E,12S,14S)-5-hydroxy-1-[(1 R,3R,4R)-4-hydroxy-3-methoxycyclohexyl]-14-methoxy-14- [(2R,3S,5R)-3-methoxy-5-methyl-6-oxooxan-2-yl]-2,4,10,12-tetramethyl-7-oxo-8-(prop-2-en-1-yl)tetradeca-1,9-dien-3-yl (2S)-1-formylpiperidine-2-carboxylate,
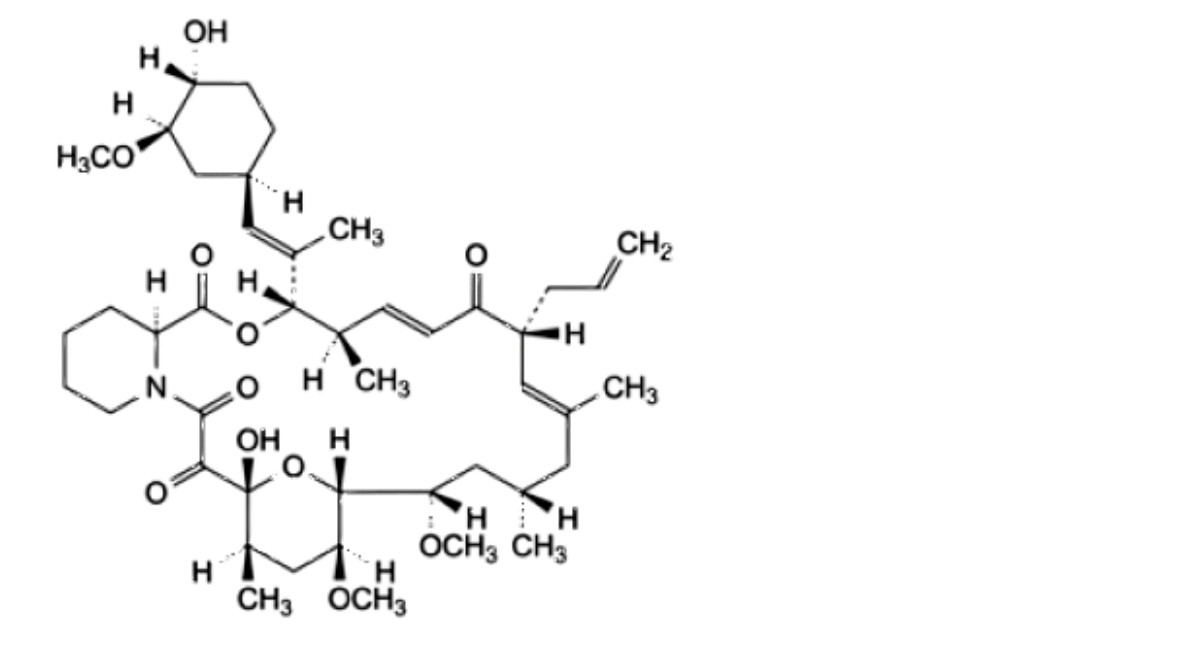
I. (3S,4R,5E,8R,9E,12S,14S,15R,16S,18R,19R,26aS)-19-hydroxy-3-[(1 E)-1-[(1 R,3R,4R)-4-hydroxy-3- methoxycyclohexyl]prop-1-en-2-yl]-14,16-dimethoxy-4,10,12,18-tetramethyl-8-(prop-2-en-1- yl)-3,4,8,11,12,13,14,15,16,17,18,19,24,25,26,26a-hexadecahydro-7R-15,19-epoxypyrido[2,1-c][1,4]oxaazacyclotricosine- 1,7,20,21(23R)-tetrone.
Ph Eur






