(Ph. Eur. monograph 0592)
C6H8O2 112.1 110-44-1
Action and use
Antimicrobial preservative.
DEFINITION
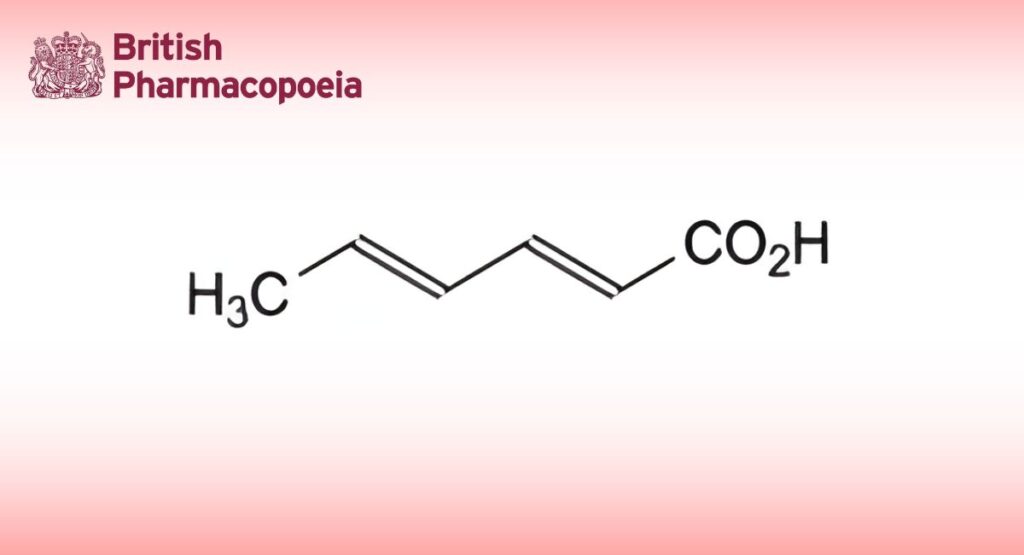
(E,E)-Hexa-2,4-dienoic acid.
Content
99.0 per cent to 101.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Slightly soluble in water, freely soluble in ethanol (96 per cent).
IDENTIFICATION
First identification: A, C.
Second identification: A, B, D.
A. Melting point (2.2.14): 132 °C to 136 °C.
B. Ultraviolet and visible absorption spectrophotometry (2.2.25).
Test solution: Dissolve 50.0 mg in water R and dilute to 250.0 mL with the same solvent. Dilute 2.0 mL of this solution to 200.0 mL with 0.1 M hydrochloric acid.
Spectral range: 230-350 nm.
Absorption maximum: At 264 nm.
Specific absorbance at the absorption maximum: 2150 to 2550.
C. Infrared absorption spectrophotometry (2.2.24).
Comparison: sorbic acid CRS.
D. Dissolve 0.2 g in 2 mL of ethanol (96 per cent) R and add 0.2 mL of bromine water R. The solution is decolorised.
TESTS
Solution S
Dissolve 1.25 g in ethanol (96 per cent) R and dilute to 25 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and colourless (2.2.2, Method II).
Aldehydes
Maximum 0.15 per cent, calculated as C2H4O.
Dissolve 1.0 g in a mixture of 30 mL of water R and 50 mL of 2-propanol R, adjust to pH 4 with 0.1 M hydrochloric acid or 0.1 M sodium hydroxide and dilute to 100 mL with water R. To 10 mL of this solution add 1 mL of decolorised fuchsin solution R and allow to stand for 30 min. Any colour in the solution is not more intense than that in a standard prepared at the same time by adding 1 mL of decolorised fuchsin solution R to a mixture of 1.5 mL of acetaldehyde standard solution (100 ppm C2H4O) R, 4 mL of 2-propanol R and 4.5 mL of water R.
Water (2.5.12)
Maximum 1.0 per cent, determined on 2.000 g.
Sulfated ash (2.4.14)
Maximum 0.2 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.1000 g in 20 mL of ethanol (96 per cent) R. Using 0.2 mL of phenolphthalein solution R as indicator, titrate with 0.1 M sodium hydroxide until a pink colour is obtained.
1 mL of 0.1 M sodium hydroxide is equivalent to 11.21 mg of C6H8O2.
STORAGE
Protected from light.