Action and use
Muscarinic M3 receptor antagonist; anticholinergic.
DEFINITION
Solifenacin Oral Solution is a solution of Solifenacin Succinate in a suitable flavoured vehicle.
The oral solution complies with the requirements stated under Oral Liquids and with the following requirements.
Content of solifenacin succinate, C27H32N2O6
95.0 to 105.0% of the stated amount.
IDENTIFICATION
In the Assay, record the UV spectrum of the principal peak in the chromatograms obtained with solutions (1) and (2) with a diode array detector in the range of 210 to 400 nm.
The UV spectrum of the principal peak in the chromatogram obtained with solution (1) is concordant with that of the peak in the chromatogram obtained with solution (2);
the retention time of the principal peak in the chromatogram obtained with solution (1) is similar to that of the peak in the chromatogram obtained with solution (2).
TESTS
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following freshly prepared solutions.
Solution A: 20 volumes of acetonitrile R1 and 30 volumes of 0.1M hydrochloric acid.
(1) To a quantity of the oral solution containing 6 mg of Solifenacin Succinate add 30 mL of solution A, shake and dilute to 50 mL with solution A.
(2) Dilute 1 volume of solution (1) to 100 volumes with solution A. Further dilute 1 volume of this solution to 5 volumes with solution A.
(3) To 10 mg of solifenacin succinate BPCRS add 1 mL of hydrogen peroxide (30%), 5 mL of 0.1M sodium hydroxide, 10 mL of water and 5 mL of acetonitrile. Stir uncovered for 10 minutes (generation of impurity I). Add 1 volume of this solution to 2.5 volumes of solution (1) and dilute to 25 volumes with solution A.
(4) 0.1% w/v of solifenacin succinate impurity standard BPCRS (containing impurities A and 1) in solution A.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (15 cm × 4.6 mm) packed with end-capped octadecylsilyl silica gel for chromatography (3.5 μm) (Waters XTerra MS C18 is suitable).
(b) Use gradient elution and the mobile phase described below.
(c) Use a flow rate of 1 mL per minute.
(d) Use a column temperature of 40°.
(e) Use an autosampler temperature of 5°.
(f) Use a detection wavelength of 210 nm.
(g) Inject 15 μL of each solution.
MOBILE PHASE
Mobile phase A: 20 volume of acetonitrile R1 and 80 volumes of a 0.05M ammonium dihydrogen orthophosphate solution previously adjusted to pH 2.4 with orthophosphoric acid.
Mobile phase B: Acetonitrile R1.
| Time (Minutes) | Mobile phase A (% v/v) | Mobile phase B (% v/v) | Comment |
| 0-25 | 95-90 | 5-10 | linear gradient |
| 25-40 | 90-75 | 10-25 | linear gradient |
| 40-41 | 75-50 | 25-50 | linear gradient |
| 41-65 | 50 | 50 | isocratic |
| 65-66 | 50-95 | 50-5 | linear gradient |
| 66-75 | 95 | 5 | re-equilibration |
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution between the peaks due to solifenacin and impurity I is at least 2.0.
CALCULATION OF IMPURITIES
For each impurity, use the concentration of solifenacin succinate in solution (2).
For the reporting threshold, use the concentration of solifenacin succinate in solution (2).
For peak identification, use solutions (3) and (4).
Solifenacin succinate retention time: about 26 minutes.
Relative retention: impurity A, about 0.2; impurity 1, about 0.5; impurity I, about 1.1.
Correction factor: impurity A, multiply by 0.7.
LIMITS
— unspecified impurities: for each impurity, not more than 0.2%;
— total impurities: not more than 0.5%;
— reporting threshold: 0.1%.
ASSAY
Carry out the method for liquid chromatography, Appendix III D, using the following solutions freshly prepared in solution A.
(1) To a weighed amount of the oral solution containing 6 mg of Solifenacin Succinate, add in 30 mL, shake and dilute to produce 50 mL. Dilute 1 volume of this solution to 10 volumes.
(2) 0.0012% w/v of solifenacin succinate BPCRS.
(3) 0.0012% w/v of solifenacin succinate BPCRS and 0.00024% w/v of propyl 4-hydroxybenzoate.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (15 cm × 4.6 mm) packed with end-capped octadecylsilyl amorphous organosilica polymer for chromatography (3.5 μm) (Waters XTerra MS C18 is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1 mL per minute.
(d) Use a column temperature of 40°.
(e) Use an autosampler temperature of 5°.
(f) Use a detection wavelength of 210 nm.
(g) Inject 25 μL of each solution.
MOBILE PHASE
30 volumes of acetonitrile R1 and 70 volumes of a 0.05M ammonium dihydrogen orthophosphate solution previously adjusted to pH 2.4 with orthophosphoric acid.
When the chromatograms are recorded under the prescribed conditions the retention time of the solifenacin is about 11 minutes.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution between the peaks due to solifenacin and propyl 4-hydroxybenzoate is at least 1.5.
DETERMINATION OF CONTENT
Determine the weight per mL of the oral solution, Appendix V G, and calculate the content of C27H32N2O6, weight in volume, using the declared content of C27H32N2O6 in solifenacin succinate BPCRS.
IMPURITIES
The impurities limited by the requirements of this monograph include impurity I listed under Solifenacin Succinate and:
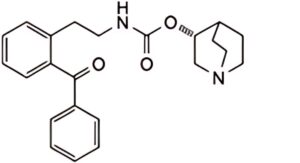
1. (+)-(R)-quinuclidin-3-yl [2-(2-benzoylphenyl)ethyl]carbamate






