(Ph. Eur. monograph 2033)
DEFINITION
Content
Minimum 50.0 per cent m/m of sodium (2S)-2-hydroxypropanoate (C3H5NaO3; Mr 112.1); 96.0 per cent to 104.0 per cent of the content of sodium lactate stated on the label, not less than 95.0 per cent of which is the (S)-enantiomer.
CHARACTERS
Appearance
Clear, colourless, slightly syrupy liquid.
Solubility
Miscible with water and with ethanol (96 per cent).
IDENTIFICATION
A. To 0.1 mL add 10 mL of water R. 5 mL of the solution gives the reaction of lactates (2.3.1).
B. It gives reaction (a) of sodium (2.3.1).
C. It complies with the limits of the assay.
TESTS
Solution S
Dilute a quantity of the substance to be examined corresponding to 40.0 g of sodium lactate to 200 mL with distilled water R.
Appearance of solution
The substance to be examined is clear (2.2.1) and not more intensely coloured than reference solution BY7 (2.2.2, Method II).
pH (2.2.3)
6.5 to 9.0 for the substance to be examined.
Reducing sugars and sucrose
To 5 mL of the substance to be examined add 2 mL of dilute sodium hydroxide solution R and 0.2 mL of copper sulfate solution R. The solution is clear and blue and remains so on boiling. Add to the hot solution 4 mL of hydrochloric acid R.
Boil for 1 min. Add 6 mL of strong sodium hydroxide solution R and heat to boiling again. The solution is clear and blue.
Methanol
Gas chromatography (2.4.24).
Limit:
— methanol: maximum 50 ppm, calculated with reference to sodium lactate, if intended for use in the manufacture of parenteral preparations, dialysis, haemodialysis or haemofiltration solutions.
Chlorides (2.4.4)
Maximum 50 ppm calculated with reference to sodium lactate.
Dilute 5 mL of solution S to 15 mL with water R.
Oxalates and phosphates
To 1 mL of the substance to be examined add 15 mL of ethanol (96 per cent) R and 2 mL of calcium chloride solution R.
Heat at 75 °C for 5 min. Any opalescence in the solution is not more intense than that of a standard prepared at the same time and in the same manner using a mixture of 1 mL of the substance to be examined, 15 mL of ethanol (96 per cent) R and 2 mL of water R.
Sulfates (2.4.13)
Maximum 100 ppm calculated with reference to sodium lactate.
To 7.5 mL of solution S, add 1.9 mL of hydrochloric acid R1 and dilute to 15 mL with distilled water R. The solution complies with the limit test for sulfates without addition of 0.5 mL of acetic acid R. Acidify the standard solution with 0.05 mL of hydrochloric acid R1 instead of 0.5 mL of acetic acid R.
Aluminium
Maximum 0.1 ppm, if intended for use in the manufacture of parenteral preparations, dialysis, haemodialysis or haemofiltration solutions.
Atomic absorption spectrometry (2.2.23, Method I). For the preparation of the solutions, use equipment that is aluminium- free or that will not release aluminium under the conditions of use (glass, polyethylene, etc).
Modifier solution: Dissolve 100.0 g of ammonium nitrate R in a mixture of 50 mL of water R and 4 mL of nitric acid R and dilute to 200 mL with water R.
Blank solution: Dilute 2.0 mL of the modifier solution to 25.0 mL with water R.
Test solution: To 5.0 g add 2.0 mL of the modifier solution and dilute to 25.0 mL with water R.
Reference solutions: Prepare the reference solutions immediately before use (0.010 ppm to 0.050 ppm of aluminium) using aluminium standard solution (200 ppm Al) R.
Source: Aluminium hollow-cathode lamp.
Wavelength: 309.3 nm.
Atomisation device: Graphite furnace.
Carrier gas: argon R.
Conditions: The device is equipped with a non-specific absorption correction system. Heat the oven to 120 °C for as many seconds as there are microlitres of solution introduced into the apparatus, then heat at 1000 °C for 30 s and finally at 2700 °C for 6 s.
Iron (2.4.9)
Maximum 10 ppm calculated with reference to sodium lactate.
Dilute 5 mL of solution S to 10 mL with water R.
ASSAY
Dissolve a quantity of the substance to be examined corresponding to 75.0 mg of sodium lactate in a mixture of 10 mL of glacial acetic acid R and 20 mL of acetic anhydride R. Allow to stand for 15 min. Add 1 mL of naphtholbenzein solution R and titrate with 0.1 M perchloric acid.
1 mL of 0.1 M perchloric acid is equivalent to 11.21 mg of C3H5NaO3.
(S)-enantiomer
Transfer a quantity of the substance to be examined corresponding to 2.50 g of sodium lactate into a 50 mL volumetric flask, dilute with about 30 mL of water R and add 5.0 g of ammonium molybdate R. Dissolve and dilute with water R to 50.0 mL. Measure the angle of optical rotation (2.2.7). Calculate the percentage content of (S)-enantiomer using the
expression:
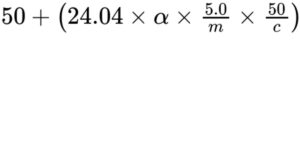
α = angle of optical rotation (absolute value);
m = mass of the substance to be examined, in grams;
c = percentage content m/m of C3H5NaO3 in the substance to be examined.
The complex of sodium (S)-lactate formed under these test conditions is laevorotatory.
LABELLING
The label states:
— where applicable, that the substance is suitable for use in the manufacture of dialysis, haemodialysis and haemofiltration solutions;
— where applicable, that the substance is suitable for use in the manufacture of parenteral preparations;
— the declared content of sodium lactate.






