(Ph. Eur. monograph 0123)
C7H5NaO2 144.1 532-32-1
Action and use
Excipient; treatment of hyperammonaemia due to urea cycle disorders; treatment of non-ketotic hyperglycinaemia.
Preparations
Sodium Benzoate Oral Solution
Sodium Benzoate Sterile Concentrate
DEFINITION
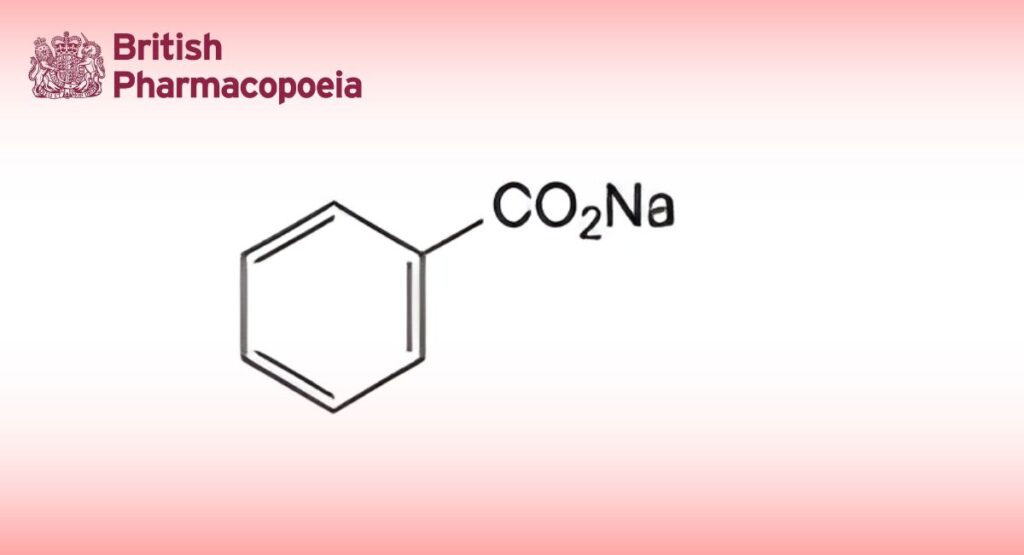
Sodium benzenecarboxylate.
Content
99.0 per cent to 100.5 per cent (dried substance).
CHARACTERS
Appearance
White or almost white, crystalline or granular powder or flakes, slightly hygroscopic.
Solubility
Freely soluble in water, sparingly soluble in ethanol (90 per cent V/V).
IDENTIFICATION
A. It gives reactions (b) and (c) of benzoates (2.3.1).
B. It gives reaction (a) of sodium (2.3.1).
TESTS
Solution S
Dissolve 10.0 g in carbon dioxide-free water R and dilute to 100 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and not more intensely coloured than reference solution Y6 (2.2.2, Method II).
Acidity or alkalinity
To 10 mL of solution S add 10 mL of carbon dioxide-free water R and 0.2 mL of phenolphthalein solution R. Not more than 0.2 mL of 0.1 M sodium hydroxide or 0.1 M hydrochloric acid is required to change the colour of the indicator.
Halogenated compounds
Maximum 200 ppm for ionised chlorine and maximum 300 ppm for total chlorine.
All glassware used must be chloride-free and may be prepared by soaking overnight in a 500 g/L solution of nitric acid R, rinsed with water R and stored full of water R. It is recommended that glassware be reserved exclusively for this test.
Test solution: To 20.0 mL of solution S add 5 mL of water R and dilute to 50.0 mL with ethanol (96 per cent) R.
Determination of ionised chlorine
In three 25 mL volumetric flasks, prepare the following solutions.
Solution (a): To 4.0 mL of the test solution add 3 mL of dilute sodium hydroxide solution R and 3 mL of ethanol (96 per cent) R. This solution is used to prepare solution A.
Solution (b): To 3 mL of dilute sodium hydroxide solution R add 2 mL of water R and 5 mL of ethanol (96 per cent) R. This solution is used to prepare solution B.
Solution (c): To 4.0 mL of chloride standard solution (8 ppm Cl) R add 6.0 mL of water R. This solution is used to prepare solution C.
In a fourth 25 mL volumetric flask, place 10 mL of water R. To each flask add 5 mL of ferric ammonium sulfate solution R5, mix and add dropwise and with swirling 2 mL of nitric acid R and 5 mL of mercuric thiocyanate solution R. Shake. Dilute the contents of each flask to 25.0 mL with water R and allow the solutions to stand in a water-bath at 20 °C for 15 min.
Measure at 460 nm in a 2 cm cell the absorbance (2.2.25) of solution A using solution B as the compensation liquid, and the absorbance of solution C using the solution obtained with 10 mL of water R as the compensation liquid. The absorbance of solution A is not greater than that of solution C.
Determination of total chlorine
Solution (a): To 10.0 mL of the test solution add 7.5 mL of dilute sodium hydroxide solution R and 0.125 g of nickel- aluminium alloy R and heat on a water-bath for 10 min. Allow to cool to room temperature, filter into a 25 mL volumetric flask and wash the filter with 3 quantities, each of 2 mL, of ethanol (96 per cent) R (a slight precipitate may form that disappears on acidification). Dilute the filtrate and washings to 25.0 mL with water R. This solution is used to prepare solution A.
Solution (b): In the same manner, prepare a similar solution replacing the test solution by a mixture of 5 mL of ethanol (96 per cent) R and 5 mL of water R. This solution is used to prepare solution B.
Solution (c): To 6.0 mL of chloride standard solution (8 ppm Cl) R add 4.0 mL of water R. This solution is used to prepare solution C.
In four 25 mL volumetric flasks, place separately 10 mL of solution (a), 10 mL of solution (b), 10 mL of solution (c) and 10 mL of water R. To each flask add 5 mL of ferric ammonium sulfate solution R5, mix and add dropwise and with swirling 2 mL of nitric acid R and 5 mL of mercuric thiocyanate solution R. Shake. Dilute the contents of each flask to 25.0 mL with water R and allow the solutions to stand in a water-bath at 20 °C for 15 min. Measure at 460 nm in a 2 cm cell the
absorbance (2.2.25) of solution A using solution B as the compensation liquid, and the absorbance of solution C using the
solution obtained with 10 mL of water R as the compensation liquid. The absorbance of solution A is not greater than that of solution C.
Loss on drying (2.2.32)
Maximum 2.0 per cent, determined on 1.00 g by drying in an oven at 105 °C.
ASSAY
Dissolve 0.250 g in 20 mL of anhydrous acetic acid R, heating to 50 °C if necessary. Cool. Using 0.05 mL of naphtholbenzein solution R as indicator, titrate with 0.1 M perchloric acid until a green colour is obtained.
1 mL of 0.1 M perchloric acid is equivalent to 14.41 mg of C7H5NaO2.