(Ph. Eur. monograph 1994)
Action and use
Used in the treatment of rheumatoid arthritis.
Preparation
Sodium Aurothiomalate Injection
DEFINITION
Mixture of monosodium and disodium salts of (2RS)-2 (aurosulfanyl)butanedioic acid.
Content
— gold (Au; Ar 197.0): 44.5 per cent to 46.0 per cent (dried substance);
— sodium (Na; Ar 22.99): 10.8 per cent to 11.8 per cent (dried substance).
CHARACTERS
Appearance
Fine, pale yellow, hygroscopic powder.
Solubility
Very soluble in water, practically insoluble in ethanol (96 per cent) and in methylene chloride.
IDENTIFICATION
A. Dissolve 20 mg in water R and dilute to 2 mL with the same solvent. Add 2 mL of strong hydrogen peroxide solution R and 1 mL of sodium hydroxide solution R. Carefully heat to boiling and boil for 30 s. A precipitate is produced that appears brownish-black by reflected light and bluish-green by transmitted light.
B. To 20 mg add 2 mL of water R. The solution gives reaction (a) of sodium (2.3.1).
C. Ignite 100 mg, dissolve the residue in hydrochloric acid R and dilute to 10 mL with the same acid. Allow to stand. 5 mL of the clear supernatant gives reaction (a) of sulfates (2.3.1).
TESTS
Appearance of solution
Dissolve 1.0 g in water R and dilute to 10 mL with the same solvent. Filter, seal in an ampoule and heat at 100 °C for 1 h. Cool and dilute the contents of the ampoule to 100 mL with water R. The solution remains clear and is not more intensely coloured than a 0.100 g/L solution of potassium ferricyanide R.
pH (2.2.3)
6.0 to 7.0.
Dissolve 1.0 g in carbon dioxide-free water R and dilute to 10 mL with the same solvent.
Related substances
Liquid chromatography (2.2.29). Prepare the solutions immediately before use.
Test solution: Dissolve 0.200 g of the substance to be examined in water R and dilute to 100.0 mL with the same solvent.
Reference solution (a): Dissolve 10.0 mg of fumaric acid R and 100.0 mg of thiomalic acid R in water R and dilute to 100.0 mL with the same solvent. Dilute 1.0 mL of this solution to 25.0 mL with water R.
Reference solution (b): Dissolve 12.0 mg of thiomalic acid R in water R and dilute to 100.0 mL with the same solvent.
Reference solution (c): Dissolve 10.0 mg of maleic acid R in water R and dilute to 100.0 mL with the same solvent. Dilute 1.0 mL of this solution to 25.0 mL with water R.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: base-deactivated end-capped octadecylsilyl silica gel for chromatography R (5 μm).
Mobile phase: Mix 90 volumes of a 10.5 g/L solution of phosphoric acid R, 100 volumes of methanol R2 and 810 volumes of water R.
Flow rate: 1.0 mL/min.
Detection: Spectrophotometer at 205 nm.
Injection: 10 μL.
Run time: Twice the retention time of impurity C.
Relative retention: With reference to impurity C (retention time = about 8 min): impurity A = about 0.4; impurity B = about 0.6. Aurothiomalate does not elute under the chromatographic conditions described.
System suitability: Reference solution (a):
— resolution: minimum 5.0 between the peaks due to impurities B and C.
Limits:
— impurity A: not more than the area of the principal peak in the chromatogram obtained with reference solution (c) (0.2 per cent);
— impurity B: not more than the area of the corresponding peak in the chromatogram obtained with reference solution (a) (0.2 per cent);
— impurity C: not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (6.0 per cent).
Maximum 8.0 per cent.
Test solution: Dissolve 0.50 g of the substance to be examined in water R and dilute to 10 mL with the same solvent.
Reference solution (a): Dilute 0.80 g of glycerol R to 100.0 mL with water R.
Reference solution (b): To 2.5 mL of reference solution (a) add 7.5 mL of water R.
Reference solution (c): To 5.0 mL of reference solution (a) add 5.0 mL of water R.
Reference solution (d): To 7.5 mL of reference solution (a) add 2.5 mL of water R.
Blank solution: 10 mL of water R.
To the test solution, reference solutions (b), (c) and (d) and the blank solution, add 2.5 mL of a freshly prepared 235 g/L solution of sodium hydroxide R and mix. Add dropwise in 0.2 mL increments a 38.0 g/L solution of cupric chloride R, shaking vigorously after each addition, until the solutions become slightly turbid. Then add 0.2 mL of the 38.0 g/L solution,of cupric chloride R. Stopper and shake vigorously for 1 min. Dilute to 25.0 mL with water R and mix. Centrifuge for 2 min. Measure the absorbance (2.2.25) of the supernatant of a 1 cm layer at 635 nm. Use the solution prepared from the blank solution as the compensation liquid. Draw a calibration curve and calculate the content of glycerol in the sample.
Loss on drying (2.2.32)
Maximum 2.0 per cent, determined on 1.000 g by drying in a desiccator at a pressure not exceeding 0.7 kPa for 24 h.
ASSAY
Gold
Heat 0.2 g with 10 mL of sulfuric acid R and continue to boil gently until a clear, pale yellow liquid is produced. Cool, add about 1 mL of nitric acid R dropwise and boil for 1 h. Cool, dilute to 70 mL with water R, boil for 5 min and filter. Wash the residue of gold with water R at 60 °C. Dry and ignite at a temperature of at least 600 °C for 3 h. Weigh the residue and calculate the percentage content of Au.
Sodium
Evaporate to dryness the filtrate and washings obtained in the assay for gold, moisten with sulfuric acid R and ignite at 600 ± 50 °C for 3 h.
1.000 g of residue is equivalent to 0.324 g of Na.
STORAGE
In an airtight container.
IMPURITIES
Specified impurities A, B, C.

A. (Z)-butenedioic acid (maleic acid),
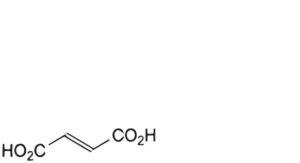
B. (E)-butenedioic acid (fumaric acid),
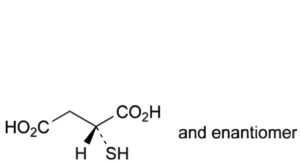
C. (2RS)-2-sulfanylbutanedioic acid (thiomalic acid).






