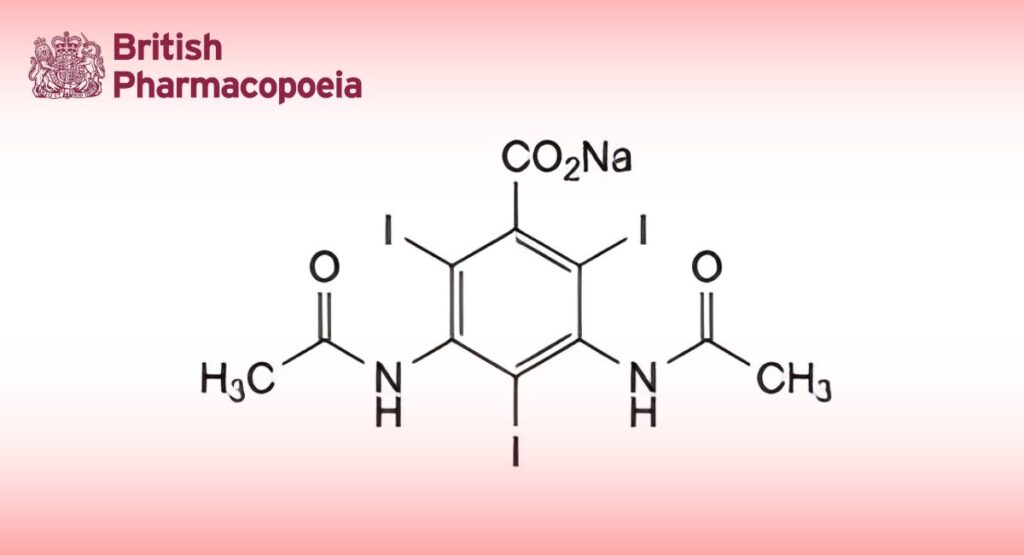
(Ph. Eur. monograph 1150)
C11H8I3N2NaO4 636 737-31-5
Action and use
Iodinated contrast medium.
DEFINITION
Sodium amidotrizoate contains not less than 98.0 per cent and not more than the equivalent of 101.0 per cent of sodium 3,5-bis(acetylamino)-2,4,6-tri-iodobenzoate, calculated with reference to the anhydrous substance.
CHARACTERS
A white or almost white powder, freely soluble in water, slightly soluble in ethanol (96 per cent), practically insoluble in acetone.
It melts at about 261 °C with decomposition.
IDENTIFICATION
First identification: A, D.
Second identification: B, C, D.
A. Examine by infrared absorption spectrophotometry (2.2.24), comparing with the spectrum obtained with sodium amidotrizoate CRS. Dry both the substance to be examined and the reference substance at 100 °C to 105 °C for 3 h.
B. Examine the chromatograms obtained in the test for related substances. The principal spot in the chromatogram obtained with test solution (b) is similar in position and size to the principal spot in the chromatogram obtained with reference solution (b).
C. Heat 50 mg gently in a small porcelain dish over a naked flame. Violet vapour is evolved.
D. It gives reaction (a) of sodium (2.3.1).
TESTS
Solution S
Dissolve 10 g in carbon dioxide-free water R and dilute to 20 mL with the same solvent.
Appearance of solution
Dilute 1 mL of solution S to 10 mL with water R. The solution is clear (2.2.1) and colourless (2.2.2, Method II).
pH (2.2.3)
The pH of solution S is 7.5 to 9.5.
Related substances
Examine by thin-layer chromatography (2.2.27), using a TLC silica gel GF254 plate R. Prepare the solutions in subdued light and develop the chromatograms protected from light.
Test solution (a): Dissolve 0.50 g of the substance to be examined in a 3 per cent V/V solution of ammonia R in methanol R and dilute to 10 mL with the same solution.
Test solution (b): Dilute 1 mL of test solution (a) to 10 mL with a 3 per cent V/V solution of ammonia R in methanol R.
Reference solution (a): Dilute 1 mL of test solution (b) to 50 mL with a 3 per cent V/V solution of ammonia R in methanol R.
Reference solution (b): Dissolve 50 mg of sodium amidotrizoate CRS in a 3 per cent V/V solution of ammonia R in methanol R and dilute to 10 mL with the same solution.
Apply separately to the plate 2 μL of each solution. Develop over a path of 15 cm using a mixture of 20 volumes of anhydrous formic acid R, 25 volumes of methyl ethyl ketone R and 60 volumes of toluene R. Allow the plate to dry until the solvents have evaporated and examine in ultraviolet light at 254 nm. Any spot in the chromatogram obtained with test solution (a), apart from the principal spot, is not more intense than the spot in the chromatogram obtained with reference solution (a) (0.2 per cent).
Free aromatic amines
Maintain the solutions and reagents in iced water, protected from light To 0.50 g in a 50 mL volumetric flask add 15 mL of water R. Shake and add 1 mL of dilute sodium hydroxide solution R. Cool in iced water, add 5 mL of a freshly prepared 5 g/L solution of sodium nitrite R and 12 mL of dilute hydrochloric acid R. Shake gently and allow to stand for exactly 2 min after adding the hydrochloric acid. Add 10 mL of a 20 g/L solution of ammonium sulfamate R. Allow to stand for 5 min, shaking frequently, and add 0.15 mL of a 100 g/L solution of α-naphthol R in ethanol (96 per cent) R. Shake and allow to stand for 5 min. Add 3.5 mL of buffer solution pH 10.9 R, mix and dilute to 50.0 mL with water R. The absorbance (2.2.25), measured within 20 min at 485 nm using as the compensation liquid a solution prepared at the same time and in the same manner but omitting the substance to be examined, is not greater than 0.30.
Free iodine and iodides
Not more than 50 ppm. Dissolve 1.0 g in distilled water R and dilute to 10 mL with the same solvent. Add dropwise dilute nitric acid R until the precipitation is complete, then add 3 mL of dilute nitric acid R. Filter and wash the precipitate with 5 mL of water R. Collect the filtrate and washings. Add 1 mL of strong hydrogen peroxide solution R and 1 mL of
methylene chloride R. Shake. The lower layer is not more intensely coloured than a reference solution prepared simultaneously and in the same manner, using a mixture of 5 mL of iodide standard solution (10 ppm I) R, 3 mL of dilute nitric acid R and 15 mL of water R.
Water (2.5.12)
Not more than 11.0 per cent, determined on 0.400 g by the semi-micro determination of water.
ASSAY
To 0.150 g in a 250 mL round-bottomed flask add 5 mL of strong sodium hydroxide solution R, 20 mL of water R, 1 g of zinc powder R and a few glass beads. Boil under a reflux condenser for 30 min. Allow to cool and rinse the condenser with 20 mL of water R, adding the rinsings to the flask. Filter through a sintered-glass filter (2.1.2) and wash the filter with several quantities of water R. Collect the filtrate and washings. Add 40 mL of dilute sulfuric acid R and titrate immediately with 0.1 M silver nitrate. Determine the end-point potentiometrically (2.2.20).
1 mL of 0.1 M silver nitrate is equivalent to 21.20 mg of C11H8I3N2NaO4.
STORAGE
Store protected from light.
IMPURITIES
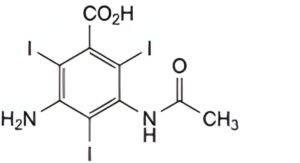
A. 3-acetylamino-5-amino-2,4,6-tri-iodobenzoic acid,
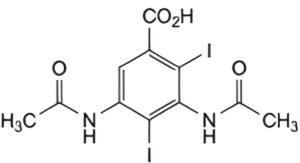
B. 3,5-bis(acetylamino)-2,4-di-iodobenzoic acid.