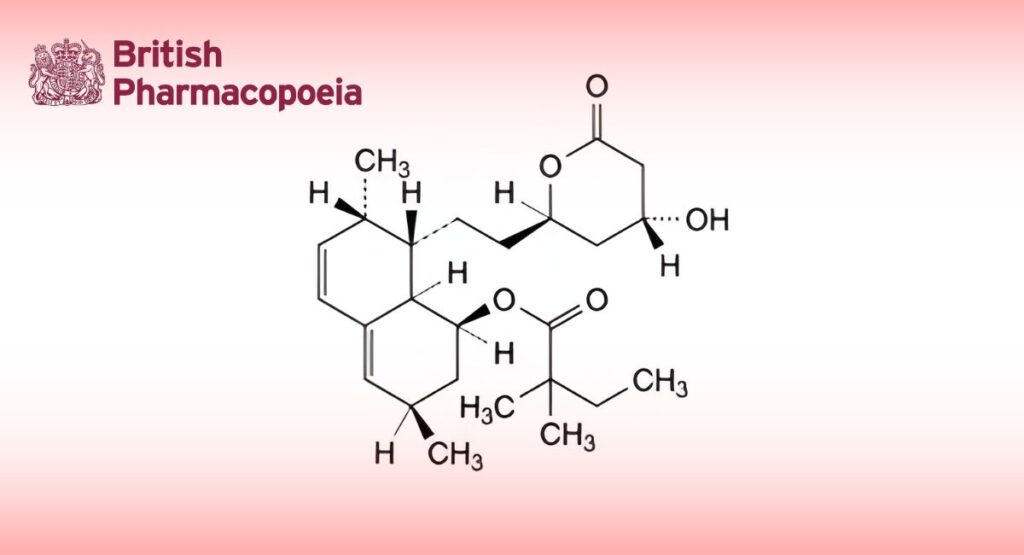
(Ph. Eur. monograph 1563)
C25H38O5 418.6 79902-63-9
Action and use
HMG Co-A reductase inhibitor; lipid-regulating drug.
Preparations
Simvastatin Tablets
Simvastatin Oral Suspension
DEFINITION
(1S,3R,7S,8S,8aR)-8-[2-[(2R,4R)-4-Hydroxy-6-oxooxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2,2-dimethylbutanoate.
Content
97.0 per cent to 102.0 per cent (dried substance).
A suitable antioxidant may be added.
CHARACTERS
Appearance
White or almost white, crystalline powder.
Solubility
Practically insoluble in water, very soluble in methylene chloride, freely soluble in ethanol (96 per cent).
IDENTIFICATION
A. Specific optical rotation (see Tests).
B. Infrared absorption spectrophotometry (2.2.24).
Comparison: simvastatin CRS.
TESTS
Appearance of solution
The solution is clear (2.2.1) and not more intensely coloured than reference solution BY7 (2.2.2, Method II).
Dissolve 0.2 g in methanol R and dilute to 20 mL with the same solvent.
Specific optical rotation (2.2.7)
+ 285 to + 300 (dried substance).
Dissolve 0.125 g in acetonitrile R and dilute to 25.0 mL with the same solvent.
Related substances
Liquid chromatography (2.2.29). Prepare the solutions immediately before use.
Solvent mixture: acetonitrile R, water R (50:50 V/V).
Test solution (a): Dissolve 50.0 mg of the substance to be examined in the solvent mixture and dilute to 25.0 mL with the solvent mixture.
Test solution (b): Dilute 1.0 mL of test solution (a) to 10.0 mL with the solvent mixture.
Reference solution (a): Dilute 1.0 mL of test solution (a) to 100.0 mL with the solvent mixture. Dilute 1.0 mL of this solution to 10.0 mL with the solvent mixture.
Reference solution (b): Dissolve 10.0 mg of simvastatin CRS in the solvent mixture and dilute to 5.0 mL with the solvent mixture. Dilute 1.0 mL of the solution to 10.0 mL with the solvent mixture.
Reference solution (c): Dissolve 2 mg of simvastatin for system suitability CRS (containing impurities A, B, C, D, E, F, G, I and J) in 1 mL of the solvent mixture.
Reference solution (d): Dissolve 4.0 mg of simvastatin impurity K CRS in the solvent mixture and dilute to 100.0 mL with the solvent mixture. Dilute 1.0 mL of the solution to 5.0 mL with the solvent mixture.
Column:
— size: l = 0.15 m, Ø = 2.1 mm;
— stationary phase: end-capped extra-dense bonded octadecylsilyl silica gel for chromatography R (3.5 μm);
— temperature: 35 °C.
Mobile phase:
— mobile phase A: acetonitrile R1, 0.1 per cent V/V solution of phosphoric acid R (40:60 V/V);
— mobile phase B: 0.1 per cent V/V solution of phosphoric acid R, acetonitrile R1 (5:95 V/V);
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 4 | 100 | 0 |
| 4 – 5 | 100 → 80 | 0 → 20 |
| 5 – 33 | 80 → 60 | 20 → 40 |
| 33 – 34 | 60 → 0 | 40 → 100 |
| 34 – 45 | 0 | 100 |
Flow rate: 0.4 mL/min.
Detection: Spectrophotometer at 238 nm and, for impurity K, at 200 nm.
Autosampler: Set at 8 °C.
Injection: 5 μL of test solution (a) and reference solutions (a), (c) and (d).
Identification of impurities: Use the chromatogram supplied with simvastatin for system suitability CRS and the chromatogram obtained with reference solution (c) to identify the peaks due to impurities A, B, C, D, E, F, G, I and J; use the chromatogram obtained with reference solution (d) to identify the peak due to impurity K.ư
Relative retention: With reference to simvastatin (retention time = about 19 min): impurity I = about 0.67; impurity A = about 0.69; impurity E = about 0.81; impurity F = about 0.83; impurity G = about 0.9; impurity K = about 1.2; impurity B = about 1.69; impurity J = about 1.74; impurity C = about 1.76; impurity D = about 2.3.
System suitability: Reference solution (c):
— peak-to-valley ratio: minimum 1.5, where Hp = height above the baseline of the peak due to impurity F and Hv = height above the baseline of the lowest point of the curve separating this peak from the peak due to impurity E; minimum 1.5, where Hp = height above the baseline of the peak due to impurity C and Hv = height above the baseline of the lowest point of the curve separating this peak from the peak due to impurity J.
Calculation of percentage contents:
— for impurity K, use the concentration of impurity K in reference solution (d) and the peak areas recorded at 200 nm;
— for impurities other than K, use the concentration of simvastatin in reference solution (a) and the peak areas recorded at 238 nm.
Limits:
— impurities E, F: for each impurity, maximum 0.5 per cent;
— sum of impurities A and I: maximum 0.4 per cent;ư
— impurity D: maximum 0.4 per cent;
— impurity K at 200 nm: maximum 0.4 per cent;
— impurities B, C: for each impurity, maximum 0.3 per cent;
— impurities G, J: for each impurity, maximum 0.2 per cent;
— unspecified impurities: for each impurity, maximum 0.10 per cent;
— total: maximum 3.0 per cent;
— reporting threshold: 0.05 per cent.
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in vacuo at 60 °C for 3 h.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modifications.
Mobile phase:
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 14 | 70 | 30 |
| 14 – 15 | 70 → 0 | 30 → 100 |
| 15 – 20 | 0 | 100 |
Injection: Test solution (b) and reference solution (b).
Retention time: Simvastatin = about 8 min.
Calculate the percentage content of C25H38O5 taking into account the assigned content of simvastatin CRS.
STORAGE
Protected from light. If no antioxidant is present, store under nitrogen, in an airtight container.
IMPURITIES
Specified impurities A, B, C, D, E, F, G, I, J, K.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) H, M, N.
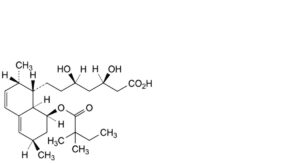
A. (3R,5R)-7-[(1S,2S,6R,8S,8aR)-8-[(2,2-dimethylbutanoyl)oxy]-2,6-dimethyl-1,2,6,7,8,8a-hexahydronaphthalen-1- yl]-3,5-dihydroxyheptanoic acid (tenivastatin),
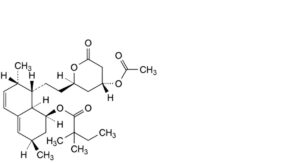
B. (1S,3R,7S,8S,8aR)-8-[2-[(2R,4R)-4-(acetyloxy)-6-oxooxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2,2-dimethylbutanoate,
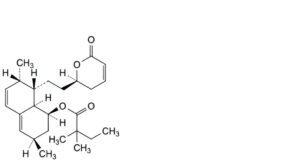
C. (1S,3R,7S,8S,8aR)-3,7-dimethyl-8-[2-[(2R)-6-oxo-3,6-dihydro-2H-pyran-2-yl]ethyl]-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2,2-dimethylbutanoate,
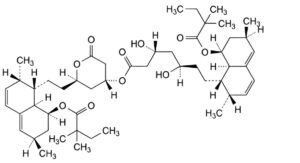
D. (2R,4R)-2-[2-[(1S,2S,6R,8S,8aR)-8-[(2,2-dimethylbutanoyl)oxy]-2,6-dimethyl-1,2,6,7,8,8a-hexahydronaphthalen-1-yl]ethyl]-6-oxooxan-4-yl (3R,5R)-7-[(1S,2S,6R,8S,8aR)-8-[(2,2-dimethylbutanoyl)oxy]-2,6-dimethyl-1,2,6,7,8,8a- hexahydronaphthalen-1-yl]-3,5-dihydroxyheptanoate,
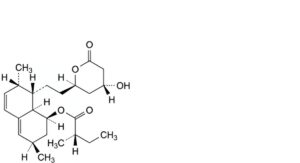
E. (1S,3R,7S,8S,8aR)-8-[2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (2S)-2-methylbutanoate (lovastatin),
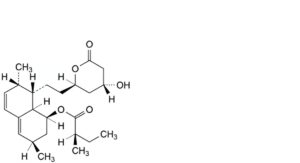
F. (1S,3R,7S,8S,8aR)-8-[2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl (2R)-2-methylbutanoate (epilovastatin),
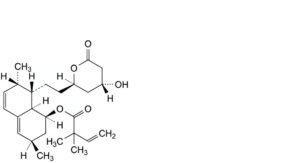
G. (1S,3R,7S,8S,8aR)-8-[2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2,2-dimethylbut-3-enoate,
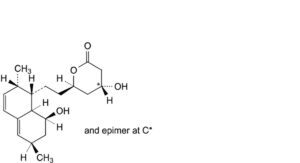
H. (4RS,6R)-4-hydroxy-6-[2-[(1S,2S,6R,8S,8aR)-8-hydroxy-2,6-dimethyl-1,2,6,7,8,8a-hexahydronaphthalen-1-yl]ethyl]oxan-2-one,
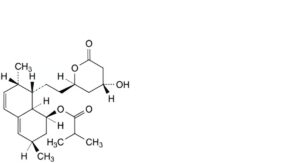
I. (1S,3R,7S,8S,8aR)-8-[2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl2-methylpropanoate,
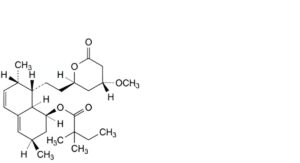
J. (1S,3R,7S,8S,8aR)-8-[2-[(2R,4R)-4-methoxy-6-oxooxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2,2-dimethylbutanoate,
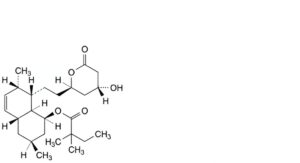
K. (1S,3S,4aR,7S,8S,8aS)-8-[2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,4,4a,7,8,8a-octahydronaphthalen-1-yl 2,2-dimethylbutanoate,
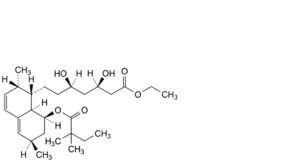
M. ethyl (3R,5R)-7-[(1S,2S,6R,8S,8aR)-8-[(2,2-dimethylbutanoyl)oxy]-2,6-dimethyl-1,2,6,7,8,8a-hexahydronaphthalen-1- yl]-3,5-dihydroxyheptanoate,
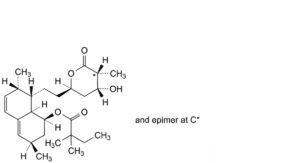
N. (1S,3R,7S,8S,8aR)-8-[2-[(2R,4R,5RS)-4-hydroxy-5-methyl-6-oxooxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a- hexahydronaphthalen-1-yl 2,2-dimethylbutanoate.