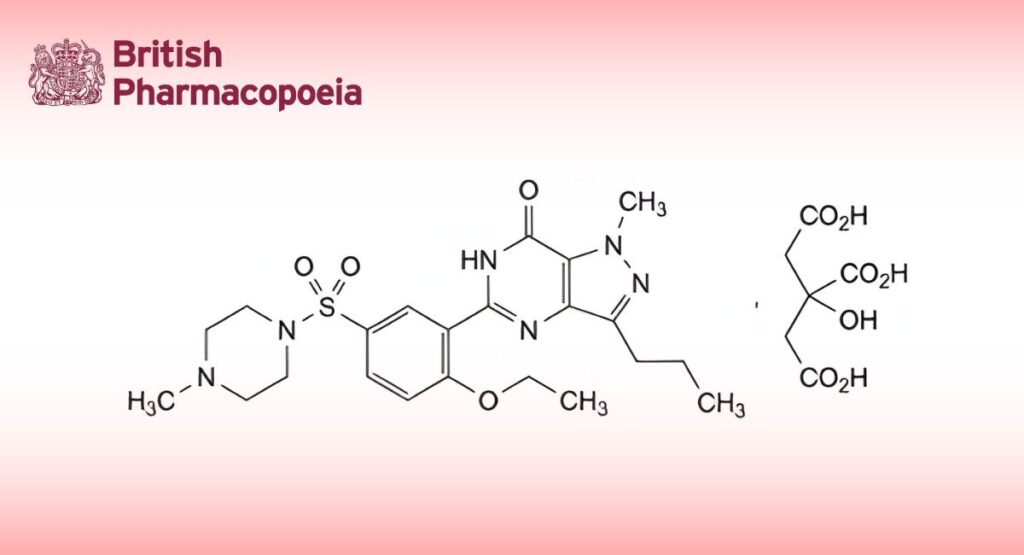
(Ph. Eur. monograph 2270)
C28H38N6O11S 667 171599-83-0
Action and use
Selective inhibitor of cyclic GMP-specific phosphodiesterase (Type V) with vasodilator action; treatment of erectile dysfunction.
Preparations
Sildenafil Tablets
Sildenafil Chewable Tablets
Sildenafil Orodispersible Tablets
Sildenafil Orodispersible Films
Sildenafil Powder for Oral Suspension
Sildenafil Injection
DEFINITION
5-[2-Ethoxy-5-(4-methylpiperazine-1-sulfonyl)phenyl]-1-methyl-3-propyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate.
Content
98.0 per cent to 102.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white, slightly hygroscopic, crystalline powder.
Solubility
Slightly soluble in water and in methanol, practically insoluble in hexane.
IDENTIFICATION
First identification: A.
Second identification: B, C.
A. Infrared absorption spectrophotometry (2.2.24).
Comparison: sildenafil citrate CRS.
B. Thin-layer chromatography (2.2.27).
Test solution: Dissolve 10 mg of the substance to be examined in methanol R and dilute to 5.0 mL with the same solvent.
Reference solution: Dissolve 10 mg of sildenafil citrate CRS in methanol R and dilute to 5.0 mL with the same solvent.
Plate: TLC silica gel F254 plate R.
Mobile phase: concentrated ammonia R, methanol R, methylene chloride R (1:14:85 V/V).
Application: 5 μL.
Development: Over 3/4 of the plate.
Drying: In air.
Detection A: Examine in ultraviolet light at 254 nm.
Results A: The principal spot in the chromatogram obtained with the test solution is similar in position and size to the principal spot in the chromatogram obtained with the reference solution.
Detection B: Treat with dilute potassium iodobismuthate solution R; examine the chromatogram in daylight.
Results B: The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with the reference solution.
C. To 0.1 g of the substance to be examined, add 10 mL of water R and 0.1 mL of concentrated ammonia R. Allow to stand for 5 min with gentle swirling. Filtrate through a paper filter. To the filtrate add 1 mL of calcium chloride solution R; the solution is clear. Boil the solution in a water bath and rub the inside of the test-tube with a glass rod. A white precipitate is formed.
TESTS
Impurity E
Thin-layer chromatography (2.2.27).
Solvent mixture: concentrated ammonia R, water R, methanol R (5:25:75 V/V/V).
Test solution: Dissolve 35.0 mg of the substance to be examined in 2.0 mL of the solvent mixture, with the aid of ultrasound if necessary.
Reference solution (a): Dissolve 7.0 mg of imidazole CRS (impurity E) in the solvent mixture and dilute to 20.0 mL with the solvent mixture. Dilute 1.0 mL of the solution to 10.0 mL with the solvent mixture.
Reference solution (b): Dilute 5.0 mL of reference solution (a) to 10.0 mL with the solvent mixture.
Reference solution (c): Mix 1 mL of the test solution and 1 mL of reference solution (a).
Plate TLC silica gel F254 plate R (2-10 μm).
Mobile phase concentrated ammonia R, ethanol (96 per cent) R, ethyl acetate R, methylene chloride R (1:20:30:50 V/V/V/V).
Application: 10 μL of the test solution and reference solutions (b) and (c) as bands of 6 mm.
Development: Over 2/3 of the plate.
Drying: At 100 °C for about 15 min.
Detection: Expose to iodine vapour until the plate is light brown and examine under ultraviolet light at 254 nm.
Retardation factors: Citrate = about 0; impurity E = about 0.25; sildenafil = about 0.4.
System suitability: Reference solution (c):
— the chromatogram shows 2 clearly separated zones.
Limit:
— impurity E: any zone due to impurity E is not more intense than the principal zone in the chromatogram obtained with reference solution (b) (0.1 per cent).
Related substances
Liquid chromatography (2.2.29).
Buffer solution: Dissolve 2.72 g of potassium dihydrogen phosphate R in 900 mL of water for chromatography R and adjust to pH 6.5 with a 120 g/L solution of potassium hydroxide R in water for chromatography R. Dilute to 1.0 L with water for chromatography R.
Solvent mixture: acetonitrile for chromatography R, mobile phase A (10:90 V/V).
Test solution (a): Dissolve 25.0 mg of the substance to be examined in the solvent mixture and dilute to 50.0 mL with the solvent mixture.
Test solution (b): Dilute 2.0 mL of test solution (a) to 50.0 mL with the solvent mixture.
Reference solution (a): Dissolve 2 mg of sildenafil impurity A CRS in test solution (a) and dilute to 10.0 mL with test solution (a). Dilute 1.0 mL of the solution to 20.0 mL with the solvent mixture.
Reference solution (b): Dissolve 5 mg of sildenafil for peak identification CRS (containing impurity D) in the solvent mixture and dilute to 10.0 mL with the solvent mixture.
Reference solution (c): Dilute 1.0 mL of test solution (a) to 100.0 mL with the solvent mixture. Dilute 1.0 mL of this solution to 10.0 mL with the solvent mixture.
Reference solution (d): Dissolve 25.0 mg of sildenafil citrate CRS in the solvent mixture and dilute to 50.0 mL with the solvent mixture. Dilute 2.0 mL of the solution to 50.0 mL with the solvent mixture.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (5 μm).
Mobile phase:
— mobile phase A: acetonitrile for chromatography R, buffer solution (20:80 V/V);
— mobile phase B: buffer solution, methanol R1, acetonitrile for chromatography R (20:20:60 V/V/V);
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 3 | 75 | 25 |
| 3 – 26 | 75 → 30 | 25 → 70 |
| 26 – 38 | 30 | 70 |
Flow rate: 1.5 mL/min.
Detection: Spectrophotometer at 230 nm.
Injection: 10 μL of test solution (a) and reference solutions (a), (b) and (c).
Identification of impurities: Use the chromatogram obtained with reference solution (a) to identify the peak due to impurity A; use the chromatogram supplied with sildenafil for peak identification CRS and the chromatogram obtained with reference solution (b) to identify the peak due to impurity D.
Relative retention: With reference to sildenafil (retention time = about 16 min): citrate = about 0.1; impurity D = about 0.15; impurity A = about 1.25.
System suitability: Reference solution (a):
— resolution: minimum 5.0 between the peaks due to sildenafil and impurity A.
Calculation of percentage contents:
— correction factor: multiply the peak area of impurity D by 0.7;
— for each impurity, use the concentration of sildenafil citrate in reference solution (c).
Limits:
— impurities A, D: for each impurity, maximum 0.15 per cent;
— unspecified impurities: for each impurity, maximum 0.10 per cent;
— total: maximum 0.5 per cent;
— reporting threshold: 0.05 per cent; disregard the peak due to citrate.
Water (2.5.12)
Maximum 2.5 per cent, determined on 0.200 g.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modification.
Injection: Test solution (b) and reference solution (d).
Calculate the percentage content of C28H38N6O11S taking into account the assigned content of sildenafil citrate CRS.
STORAGE
In airtight container.
IMPURITIES
Specified impurities A, D, E.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) B, C, F, G.
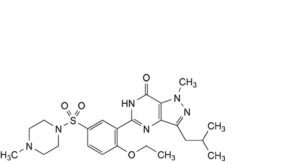
A. 5-[2-ethoxy-5-(4-methylpiperazine-1-sulfonyl)phenyl]-1-methyl-3-(2-methylpropyl)-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one,
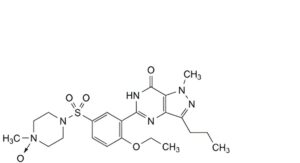
B. 1-methyl-4-[4-ethoxy-3-(1-methyl-7-oxo-3-propyl-6,7-dihydro-1H-pyrazolo[4,3-d]pyrimidin-5-yl)benzene-1-sulfonyl]piperazine 1-oxide,
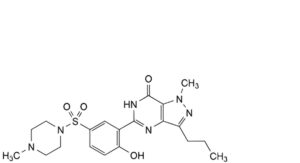
C. 5-[2-hydroxy-5-(4-methylpiperazine-1-sulfonyl)phenyl]-1-methyl-3-propyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one,
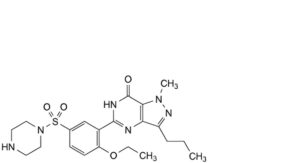
D. 4-ethoxy-3-(1-methyl-7-oxo-3-propyl-6,7-dihydro-1H-pyrazolo[4,3-d]pyrimidin-5-yl)benzene-1-sulfonic acid,

E. 1H-imidazole,
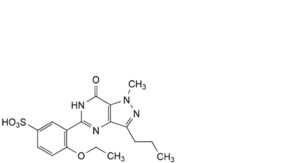
F. 5-[2-ethoxy-5-(piperazine-1-sulfonyl)phenyl]-1-methyl-3-propyl-1,6-dihydro-7H-pyrazolo[4,3-d]pyrimidin-7-one,
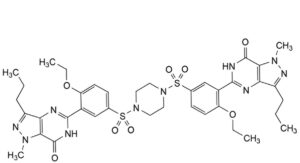
G. 2 ,6 -diethoxy-1 ,7 -dipropyl-1 H,7 H-3,5-dithia-1,7(5)-bis(pyrazolo[4,3-d]pyrimidina)-4(1,4)-piperazina-2,6(1,3)-dibenzenaheptaphan-1 ,3,3,5,5,7 -hexaone.