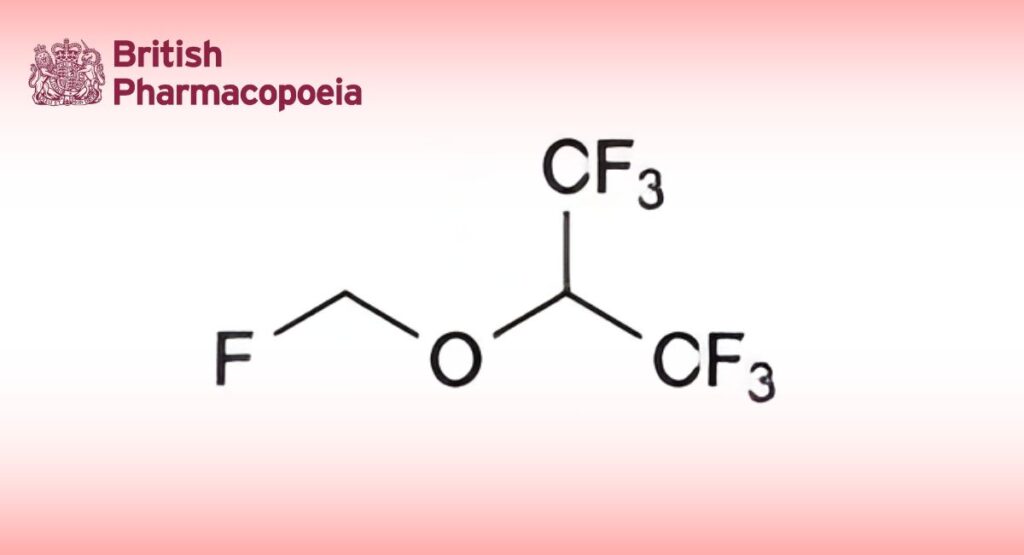
(Ph. Eur. monograph 2269)
C4H3F7O 200.1 28523-86-6
Action and use
General anaesthetic.
DEFINITION
1,1,1,3,3,3-Hexafluoro-2-(fluoromethoxy)propane.
CHARACTERS
Appearance
Clear, colourless, volatile liquid.
Solubility
Slightly soluble in water, miscible with ethanol (96 per cent).
Relative density
About 1.52.
bp
About 59 °C.
It is non-flammable.
It decomposes in the presence of Lewis acids; this decomposition is inhibited by water in sufficient quantity.
IDENTIFICATION
Infrared absorption spectrophotometry (2.2.24).
Preparation: Examine the substance in the gaseous state or in the liquid state.
Comparison: sevoflurane CRS.
TESTS
Acidity or alkalinity
Introduce 20.0 mL of the substance to be examined and 20 mL of carbon dioxide-free water R into a separating funnel, shake for 3 min and allow to stand. Collect the aqueous upper layer and add 0.2 mL of bromocresol purple solution R. Not more than 0.10 mL of 0.01 M sodium hydroxide or 0.60 mL of 0.01 M hydrochloric acid is required to change the colour of
the indicator.
Refractive index (2.2.6)
1.2745 to 1.2760.
Related substances
Gas chromatography (2.2.28).
Internal standard: methylal R.
Test solution: Introduce 20.0 mL of the substance to be examined into a vial and seal with a cap fitted with a septum. Using a microsyringe, add 5 μL of the internal standard and mix thoroughly.
Reference solution (a): Introduce 2.0 mL of heptane R into a screw-cap vial and immediately seal with a cap fitted with a septum. Using a microsyringe, add about 20 μL of the substance to be examined. Record the quantity added, in milligrams, of the substance to be examined (M2). Then, using a microsyringe, add about 20 μL of the internal standard. Record the quantity added, in milligrams, of the internal standard (M1).
Reference solution (b): sevoflurane for system suitability CRS (containing impurities A and B).
Reference solution (c): Introduce 20.0 mL of heptane R into a vial and seal with a cap fitted with a septum. Using a microsyringe, add 20 μL of the substance to be examined and mix thoroughly. Dilute 0.5 mL of this solution to 100.0 mL with heptane R.
Column:
— material: fused silica;
— size: l = 30 m, Ø = 0.32 mm;
— stationary phase: cyanopropyl(3)phenyl(3)methyl(94)polysiloxane R (film thickness 3 μm).
Carrier gas: helium for chromatography R.
Flow rate: 1.0 mL/min.
Split ratio: 1:20.
Temperature:
| Time (min) |
Temperature (°C) |
|
| Column | 0 – 10 | 40 |
| 10 – 26 | 40 → 200 | |
| 26 – 40 | 200 | |
| Injection port | 200 | |
| Detector | 225 |
Detection: Flame ionisation.
Injection: 2 μL; rinse the syringe with a solution containing heptane R before injection of the reference solutions; rinse the syringe with the substance to be examined before injection of the test solution.
Identification of impurities: Use the chromatogram supplied with sevoflurane for system suitability CRS and the chromatogram obtained with reference solution (b) to identify the peaks due to impurities A and B.
Relative retention: With reference to sevoflurane (retention time = about 6.6 min): impurity A = about 0.78; impurity B = about 0.83; internal standard = about 1.35.
System suitability: Reference solution (b):
— resolution: minimum 2.0 between the peaks due to impurities A and B.
Calculate the relative response factor (F1) for reference solution (a), using the following expression:
M1×R / M2
M1 = mass of the internal standard in reference solution (a), in milligrams;
M2 = mass of the substance to be examined in reference solution (a), in milligrams;
R = ratio of the area of the peak due to sevoflurane to the area of the peak due to the internal standard from the chromatogram obtained with reference solution (a).
Calculate the content of each impurity in the substance to be examined, in parts per million, using the following expression:
0.859×R1×250 / 1.52×F1
0.859 = relative density of the internal standard;
1.52 = relative density of sevoflurane;
R1 = ratio of the area of the peak due to the impurity to the area of the peak due to the internal standard from the chromatogram obtained with the test solution;
F1 = relative response factor for reference solution (a).
Limits:
— impurity A: maximum 25 ppm;
— impurity B: maximum 100 ppm;
— unspecified impurities: for each impurity, maximum 100 ppm;
— total: maximum 300 ppm;
— disregard limit: the area of the peak due to sevoflurane in the chromatogram obtained with reference solution (c) (5 ppm).
Fluorides
Maximum 2 μg/mL.
Potentiometry (2.2.36, Method I). Use plastic utensils throughout this test.
Buffer solution: Dissolve 0.5 g of sodium citrate R and 55 g of sodium chloride R in 350 mL of water R. Carefully add 75 g of sodium hydroxide R and shake to dissolve. Cool to room temperature and carefully add 225 mL of glacial acetic acid R while stirring. Cool and add 300 mL of 2-propanol R. Dilute with water R to 1000.0 mL. The apparent pH of this solution is between 5.0 and 5.5.
Test solution: Introduce 50.0 mL of the substance to be examined and 50.0 mL of water R into a separating funnel, shake vigorously for 3 min and allow the layers to separate completely. Dilute 25.0 mL of the upper aqueous layer to 50.0 mL with the buffer solution.
Fluoride standard solution (1000 ppm F): Dissolve 221.0 mg of sodium fluoride R, previously dried at 150 °C for 4 h, in water R. Add 1.0 mL of 0.01 M sodium hydroxide and dilute to 100.0 mL with water R.
Reference stock solutions: Dilute the fluoride standard solution (1000 ppm F) with water R to obtain solutions having known concentrations of about 5 μg, 2 μg, 0.5 μg, and 0.2 μg of fluoride per millilitre.
Reference solutions: Dilute 25.0 mL of each reference stock solution to 50.0 mL with the buffer solution.
Indicator electrode: Fluoride-selective.
Reference electrode: Silver-silver chloride.
Apparatus: Voltmeter capable of a minimum reproducibility of ± 0.2 mV.
Carry out the measurements on the reference solutions and test solution. To take measurements, transfer the solution under test to a 100 mL beaker containing a polytetrafluoroethylene-coated magnetic stirring bar, and immerse the electrodes. Allow to stir on a magnetic stirrer with an insulated top until equilibrium is attained (about 2-3 min), and record the potential. Rinse the electrodes with the buffer solution and dry, taking care to avoid damaging the crystal of the specific-ion electrode.
Calculate the concentration of fluorides using the calibration curve.
Non-volatile residue
Maximum 100 mg/L.
Transfer 10.0 mL to a tared evaporating dish, evaporate to dryness on a water-bath and dry the residue at 105 °C for 2 h.
The residue weighs a maximum of 1.0 mg.
Water (2.5.12)
Maximum 0.050 per cent m/m, determined on 10.0 mL.
STORAGE
In an airtight, stainless-steel container, protected from light.
IMPURITIES
Specified impurities A, B.
Other detectable impurities (the following substances would, if present at a sufficient level, be detected by one or other of the tests in the monograph. They are limited by the general acceptance criterion for other/unspecified impurities and/or by the general monograph Substances for pharmaceutical use (2034). It is therefore not necessary to identify these impurities for demonstration of compliance. See also 5.10. Control of impurities in substances for pharmaceutical use) C.
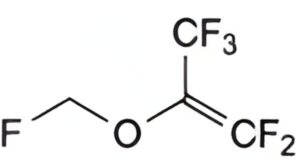
A. 1,1,3,3,3-pentafluoro-2-(fluoromethoxy)prop-1-ene,
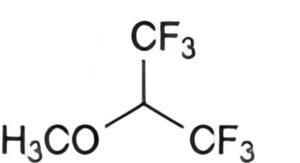
B. 1,1,1,3,3,3-hexafluoro-2-methoxypropane,
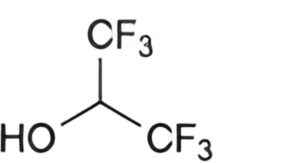
C. 1,1,1,3,3,3-hexafluoropropan-2-ol.