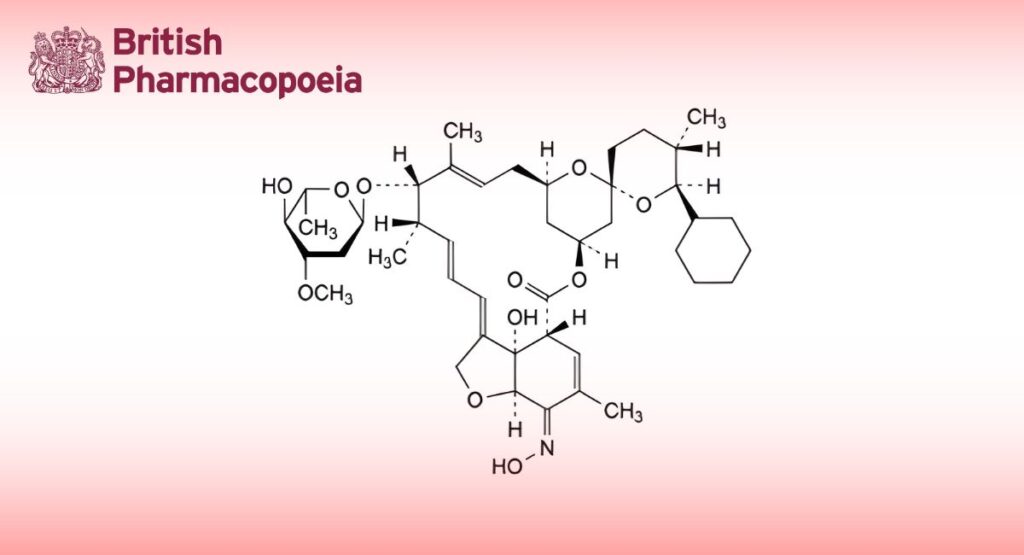
(Selamectin for Veterinary Use, Ph. Eur. monograph 2268)
C43H63NO11 770 220119-17-5
DEFINITION
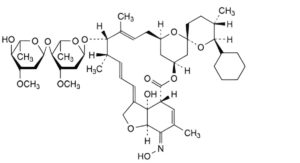
(13aS,14R,17E,17aR,2′R,4 R,42S,5′S,6E,6′S,8S,9S,10E,12E)-6′-Cyclohexyl-8-[(2,6-dideoxy-3-O-methyl-α-L-arabino- hexopyranosyl)oxy]-13a-hydroxy-17-(hydroxyimino)-16 ,5′,7,9-tetramethyl-13a,14,17,17a -tetrahydro-12 H-3-oxa-1(4,3)-[1]benzofurana-4(4,2)-oxanaspiro[cyclododecaphane-6,10,12(1 )-trien-4 ,2′-oxan]-2-one ((5Z,21R,25S)-25-cyclohexyl-25- de(butan-2-yl)-4′-O-de(2,6-dideoxy-3-O-methyl-α-L-arabino-hexopyranosyl)-5-demethoxy-5-(hydroxyimino)-22,23-
dihydroavermectin A1a).
Semi-synthetic product derived from a fermentation product.
Content
96.0 per cent to 102.0 per cent (anhydrous substance).
CHARACTERS
Appearance
White or almost white, hygroscopic powder.
Solubility
Practically insoluble in water, freely soluble in isopropyl alcohol, soluble in acetone and in methylene chloride, sparingly soluble in methanol.
IDENTIFICATION
Infrared absorption spectrophotometry (2.2.24).
Comparison: selamectin CRS.
TESTS
Related substances
Liquid chromatography (2.2.29).
Solvent mixture: water R, acetonitrile R (40:60 V/V).
Test solution: Dissolve 25 mg of the substance to be examined in the solvent mixture and dilute to 50 mL with the solvent mixture.
Reference solution (a): Dilute 1.0 mL of the test solution to 100.0 mL with the solvent mixture.
Reference solution (b): Dissolve 2.5 mg of selamectin for peak identification CRS (containing impurities A, C and D) in the solvent mixture and dilute to 5 mL with the solvent mixture.
Reference solution (c): Dissolve the contents of a vial of selamectin impurity B CRS in 1 mL of reference solution (b).
Column:
— size: l = 0.15 m, Ø = 3.9 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (4 μm);
— temperature: 30 °C.\
Mobile phase:
— mobile phase A: water for chromatography R;
— mobile phase B: acetonitrile for chromatography R;
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 28 | 40 | 60 |
| 28 – 45 | 40 → 20 | 60 → 80 |
Flow rate: 2.0 mL/min.
Detection: Spectrophotometer at 243 nm.
Injection: 20 μL.
Identification of impurities: Use the chromatogram supplied with selamectin for peak identification CRS and the chromatogram obtained with reference solution (c) to identify the peaks due to impurities A, B, C and D.
Relative retention: With reference to selamectin (retention time = about 22 min): impurity A = about 0.2; impurity B = about 0.4; impurity C = about 0.5; impurity D = about 1.7.
System suitability: Reference solution (c):
— resolution: minimum 2.5 between the peaks due to impurities B and C.
Limits:
— correction factor: for the calculation of content, multiply the peak area of impurity D by 1.5;
— impurities A, B: for each impurity, not more than twice the area of the principal peak in the chromatogram obtained with reference solution (a) (2.0 per cent);
— impurities C, D: for each impurity, not more than 1.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (1.5 per cent);
— any other impurity: for each impurity, not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (1.0 per cent);
— total: not more than 4 times the area of the principal peak in the chromatogram obtained with reference solution (a) (4.0 per cent);
— disregard limit: 0.2 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.2 per cent).
Water (2.5.12, Method A)
Maximum 7.0 per cent, determined on 0.200 g.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Liquid chromatography (2.2.29).
Test solution: Dissolve 50.0 mg of the substance to be examined in the mobile phase and dilute to 250.0 mL with the mobile phase.
Reference solution: Dissolve 50.0 mg of selamectin CRS in the mobile phase and dilute to 250.0 mL with the mobile phase.
Column:
— size: l = 0.15 m, Ø = 3.9 mm;
— stationary phase: end-capped octadecylsilyl silica gel for chromatography R (4 μm);
— temperature: 30 °C.
Mobile phase: water for chromatography R, acetonitrile for chromatography R (20:80 V/V).
Flow rate: 1.0 mL/min.
Detection: Spectrophotometer at 243 nm.
Injection: 20 μL.
Run time: Twice the retention time of selamectin.
Retention time: Selamectin = about 9 min.
Calculate the percentage content of C43H63NO11 taking into account the assigned content of selamectin CRS.
STORAGE
In an airtight container.
IMPURITIES
Specified impurities A, B, C, D.
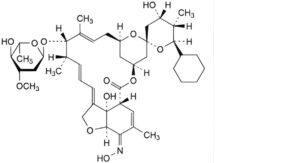
A. (5Z,21R,23S,25R)-25-cyclohexyl-25-de(butan-2-yl)-4′-O-de(2,6-dideoxy-3-O-methyl-α-L-arabino-hexopyranosyl)-5- demethoxy-23-hydroxy-5-(hydroxyimino)-22,23-dihydroavermectin A1a,
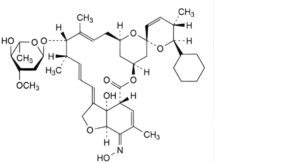
B. (5Z,25R)-25-cyclohexyl-25-de(butan-2-yl)-4′-O-de(2,6-dideoxy-3-O-methyl-α-L-arabino-hexopyranosyl)-5-demethoxy-5-(hydroxyimino)avermectin A1a,
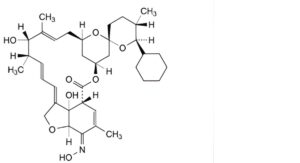
C. (5Z,21R,25S)-25-cyclohexyl-25-de(butan-2-yl)-13-O-de[2,6-dideoxy-4-O-(2,6-dideoxy-3-O-methyl-α-L-arabino- hexopyranosyl)-3-O-methyl-α-L-arabino-hexopyranosyl]-5-demethoxy-5-(hydroxyimino)-22,23-dihydroavermectin A1a,
D. (5Z,21R,25S)-25-cyclohexyl-25-de(butan-2-yl)-5-demethoxy-5-(hydroxyimino)-22,23-dihydroavermectin A1a.