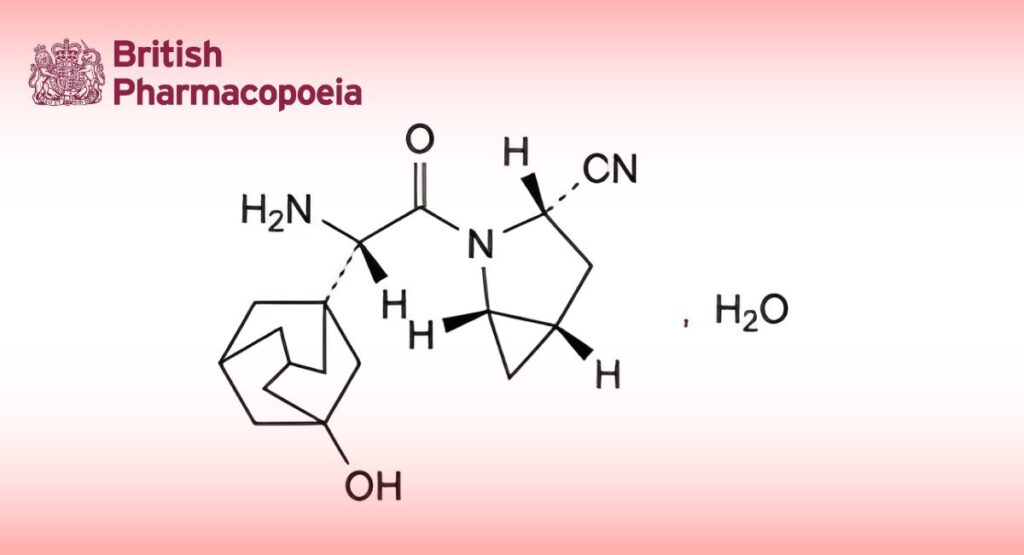
(Ph. Eur. monograph 3136)
C18H25N3O2,H2O 333.4 945667-22-1
Action and use
Dipeptidylpeptidase-4 inhibitor; treatment of type 2 diabetes mellitus.
DEFINITION
(1S,3S,5S)-2-[(S)-Amino(3-hydroxyadamantan-1-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carbonitrile monohydrate.
Content
98.0 per cent to 102.0 per cent (anhydrous substance).
PRODUCTION
It is produced by highly stereoselective methods of manufacture; consideration must be given to the formation of potential stereoisomeric impurities during the manufacturing process, and procedures must be implemented for the appropriate control of these impurities.
CHARACTERS
Appearance
White or almost white powder.
Solubility
Sparingly soluble in water, soluble in anhydrous ethanol, very slightly soluble in heptane.
IDENTIFICATION
Infrared absorption spectrophotometry (2.2.24).
Comparison: saxagliptin monohydrate CRS.
TESTS
Related substances
Liquid chromatography (2.2.29): use the normalisation procedure.
Test solution: Dissolve 54.0 mg of the substance to be examined in mobile phase A and dilute to 50.0 mL with mobile phase A.
Reference solution (a): Dissolve 54.0 mg of saxagliptin monohydrate CRS in mobile phase A and dilute to 50.0 mL with mobile phase A.
Reference solution (b): Dilute 1.0 mL of the test solution to 100.0 mL with mobile phase A. Dilute 1.0 mL of this solution to 20.0 mL with mobile phase A.
Reference solution (c): Dissolve the contents of a vial of saxagliptin impurity mixture CRS (containing impurities A and B) in 1.0 mL of mobile phase A.
Reference solution (d): Dissolve the contents of a vial of saxagliptin for system suitability CRS (containing impurity C) in 1.0 mL of mobile phase A.
Column:
— size: l = 0.15 m, Ø = 3.0 mm;
— stationary phase: end-capped extra-dense bonded octadecylsilyl silica gel for chromatography R (3.5 μm);
— temperature: 40 °C.
Mobile phase:
— mobile phase A: trifluoroacetic acid R, methanol R2, water for chromatography R (1:100:900 V/V/V);
— mobile phase B: trifluoroacetic acid R, water for chromatography R, methanol R2 (1:100:900 V/V/V);
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 2 | 100 | 0 |
| 2 – 22 | 100 → 80 | 0 → 20 |
| 22 – 42 | 80 → 0 | 20 → 100 |
Flow rate: 0.6 mL/min.
Detection: Spectrophotometer at 215 nm.
Injection: 15 μL of the test solution and reference solutions (b), (c) and (d).
Identification of impurities: Use the chromatogram obtained with reference solution (c) to identify the peaks due to impurities A and B; use the chromatogram supplied with saxagliptin for system suitability CRS and the chromatogram obtained with reference solution (d) to identify the peak due to impurity C.
Relative retention: With reference to saxagliptin (retention time = about 21 min): impurity A = about 0.73; impurity B = about 0.75; impurity C = about 1.1.
System suitability:
— peak-to-valley ratio: minimum 1.4, where Hp = height above the baseline of the peak due to impurity C and Hv = height above the baseline of the lowest point of the curve separating this peak from the peak due to saxagliptin in the chromatogram obtained with reference solution (d); minimum 2.0, where Hp = height above the baseline of the peak due to impurity B and Hv = height above the baseline of the lowest point of the curve separating this peak from the peak due to impurity A in the chromatogram obtained with reference solution (c).
Calculation of percentage content:
— correction factor: multiply the peak area of impurity A by 0.7.
Limits:
— impurities A, B, C: for each impurity, maximum 0.15 per cent;
— unspecified impurities: for each impurity, maximum 0.10 per cent;
— total: maximum 0.50 per cent;
— reporting threshold: 0.05 per cent (reference solution (b)).
Water (2.5.12)
5.2 per cent to 6.1 per cent, determined on 0.100 g.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances, with the following modifications.
Injection: Test solution and reference solution (a).
System suitability: Reference solution (a):
— symmetry factor: maximum 6.0.
Calculate the percentage content of C18H25N3O2 taking into account the assigned content of saxagliptin monohydrate CRS.
STORAGE
At a temperature of 2 °C to 8 °C.
IMPURITIES
Specified impurities A, B, C.
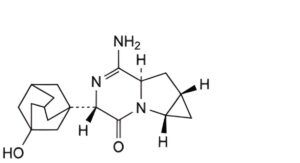
A. (1aS,4S,6aR,7aS)-6-amino-4-(3-hydroxyadamantan-1-yl)-1,1a,4,6a,7,7a-hexahydro-3H-cyclopropa[4,5]pyrrolo[1,2-a]pyrazin-3-one,
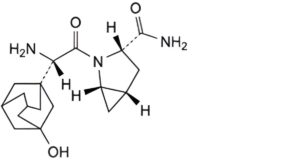
B. (1S,3S,5S)-2-[(S)-amino(3-hydroxyadamantan-1-yl)acetyl]-2-azabicyclo[3.1.0]hexane-3-carboxamide,
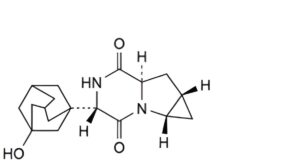
C. (1aS,4S,6aR,7aS)-4-(3-hydroxyadamantan-1-yl)hexahydro-1H-cyclopropa[4,5]pyrrolo[1,2-a]pyrazine-3,6-dione.