Action and use
Beta2-adrenoceptor agonist; bronchodilator.
DEFINITION
Salmeterol Inhalation Powder, pre-metered consists of Salmeterol Xinafoate in microfine powder or equivalent either alone or combined with a suitable carrier. The pre-metered unit is loaded into a dry-powder inhaler to generate an aerosol.
The inhalation powder, pre-metered complies with the requirements stated under Preparations for Inhalation and with the following requirements.
PRODUCTION
The size of aerosol particles to be inhaled is controlled so that a consistent portion is deposited in the lungs. The fine-particle characteristics of preparations for inhalation are determined using the method described in Appendix XII C7.
Preparations for inhalation: Aerodynamic Assessment of Fine Particles. The test and limits should be agreed with the competent authority.
The water content is controlled to ensure the performance of the product as justified and authorised by the competent authority.
Content of salmeterol, C25H37NO4
92.5 to 107.5% of the stated amount of the pre-metered unit.
IDENTIFICATION
A. Dissolve the contents of a pre-metered unit in 30 volumes of water and 70 volumes of methanol. The light absorption of this solution, Appendix II B, is in the range 210 to 350 nm and is concordant with that of a 0.0004% w/v solution of
salmeterol xinafoate EPCRS in the same solvent.
B. In the test for Uniformity of delivered dose, the retention time of the principal peak in the chromatogram obtained with solution (1) is similar to that of the peak due to salmeterol in the chromatogram obtained with solution (2).
TESTS
Uniformity of delivered dose
Complies with the requirements stated under Inhalation Powders using the following method of analysis. Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
Solution A 30 volumes of water and 70 volumes of methanol.
(1) Collect single doses of the preparation being examined using the procedure described under Inhalation Powders, Uniformity of delivered dose and dissolve the collected dose in sufficient solution A to produce a solution containing the
equivalent of 0.00025% w/v of salmeterol.
(2) 0.0004% w/v of salmeterol xinafoate EPCRS in solution A.
(3) 0.0005% w/v of salmeterol xinafoate for system suitability EPCRS in solution A.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (20 cm × 4.6 mm) packed with octadecylsilyl silica gel (5μm) (Hypersil ODS C18 is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1.5 mL per minute.
(d) Use a column temperature of 40°.
(e) Use a detection wavelength of 276 nm.
(f) Inject 200 μL of each solution.
MOBILE PHASE
0.5 volumes of formic acid, 35 volumes of 0.15M ammonium acetate and 65 volumes of methanol.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (3), the peak-to-valley ratio is at least 1.1, where Hp is the height above the baseline of the peak due to impurity E and Hv is the height above the baseline of the lowest point of the curve separating this peak from the peak due to salmeterol.
DETERMINATION OF CONTENT
Calculate the content of salmeterol, C25H37NO4, per delivered dose using the declared content of C36H45NO7 in salmeterol xinafoate EPCRS. Each mg of C36H45NO7 is equivalent to 0.6880 mg of C25H37NO4. Repeat the procedure as described for pre-metered systems under Powders for Inhalation, Uniformity of delivered dose.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
Solution A 30 volumes of water and 70 volumes of methanol.
(1) Dissolve a quantity of the powder for inhalation containing the equivalent of 0.6 mg of salmeterol in solution A, mix with the aid of ultrasound and dilute to 100 mL with solution A.
(2) Dilute 1 volume of solution (1) to 100 volumes with solution A.
(3) 0.00011% w/v of salmeterol xinafoate impurity 1 BPCRS in solution A.
(4) 0.0005% w/v of salmeterol xinafoate for system suitability EPCRS in solution A.
(5) Dilute 1 volume of solution (2) to 10 volumes with solution A.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (20 cm × 4.6 mm) packed with octadecylsilyl silica gel (5μm) (Hypersil ODS is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1.5 mL per minute.
(d) Use a column temperature of 40°.
(e) Use a detection wavelength of 276 nm. At twice the retention time of salmeterol, change the wavelength to 254 nm to enable detection of salmeterol xinafoate impurity 1.
(f) Inject 200 μL of each solution.
(g) Allow the chromatography to proceed for 3 times the retention time of salmeterol.
MOBILE PHASE
0.5 volumes of formic acid, 35 volumes of 0.15M ammonium acetate and 65 volumes of methanol When the chromatograms are recorded under the prescribed conditions, the relative retentions with reference to salmeterol (retention time about 5 minutes) are: impurity A, about 0.4; xinafoic acid, about 0.6; impurity C, about 0.7; impurity E, about 0.95; impurity F, about 1.4 and impurity 1, about 2.6.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (4), the peak-to-valley ratio is at least 1.1, where Hp is the height above the baseline of the peak due to impurity E and Hv is the height above the baseline of the lowest point of the curve separating this peak from the peak due to salmeterol.
LIMITS
In the chromatogram obtained with solution (1):
the area of any peak corresponding to impurity 1 is not greater than the area of the principal peak in the chromatogram obtained with solution (3) (3.0%);
the area of any other secondary peaks is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (1.0%);
the sum of the areas of any other secondary peaks is not greater than twice the principal peak in the chromatogram obtained with solution (2) (2.0%).
Disregard any peak with an area less than the area of the principal peak in the chromatogram obtained with solution (5) (0.1%).
ASSAY
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
Solution A 30 volumes of water and 70 volumes of methanol.
(1) Dissolve a quantity of the mixed contents of 20 pre-metered units equivalent to 0.6 mg of salmeterol with solution A, mix with the aid of ultrasound, add sufficient solution A to produce 100 mL and filter.
(2) 0.0009% w/v of salmeterol xinafoate EPCRS in solution A.
CHROMATOGRAPHIC CONDITIONS
The chromatographic conditions described under Uniformity of delivered dose may be used.
DETERMINATION OF CONTENT
Calculate the content of salmeterol, C25H37NO4, per delivered dose using the declared content of C36H45NO7 in salmeterol xinafoate EPCRS. Each mg of C36H45NO7 is equivalent to 0.6880 mg of C25H37NO4.
LABELLING
The quantity of active ingredient is stated in terms of the equivalent metered dose.
IMPURITIES
The impurities limited by the requirements of this monograph include A, C, E and F listed under Salmeterol Xinafoate and:
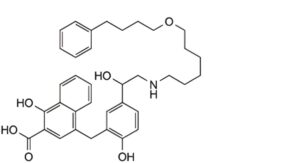
1. rac-1-hydroxy-4-{[2-hydroxy-5-(1-hydroxy-2-{6-(4-phenylbutoxy)hexyl]amino}ethyl)phenyl]methyl}naphthalene-2-carboxylic acid.






