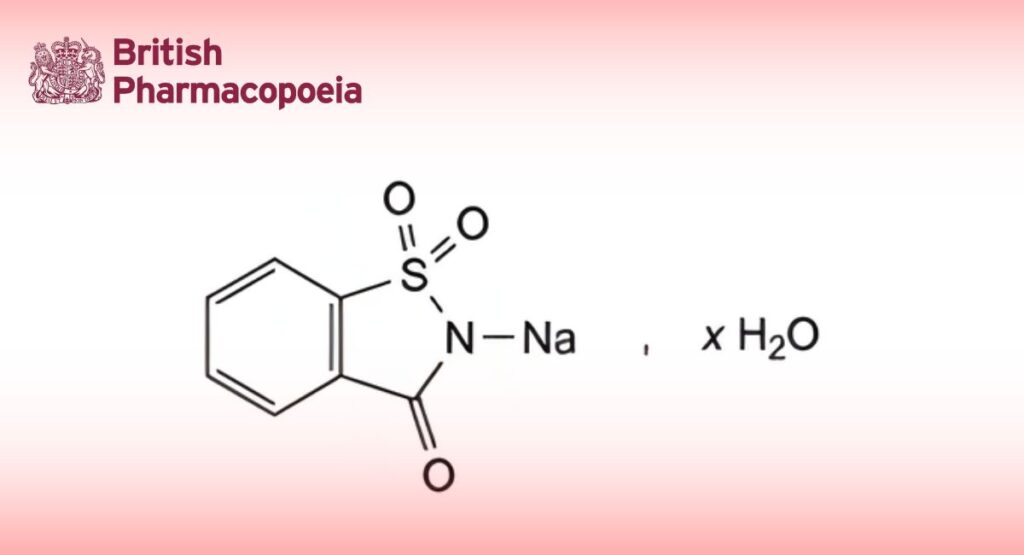
Soluble Saccharin
(Ph. Eur. monograph 0787)
C7H4NNaO3S,xH2O 205.2 (anhydrous substance)
Anhydrous saccharin sodium 128-44-9
Action and use
Sweetening agent.
DEFINITION
2-Sodio-1,2-benzisothiazol-3(2H)-one 1,1-dioxide.
Content
99.0 per cent to 101.0 per cent (anhydrous substance).
It may be anhydrous or contain a variable quantity of water.
CHARACTERS
Appearance
White or almost white, crystalline powder or colourless crystals, efflorescent in dry air.
Solubility
Freely soluble in water, sparingly soluble in ethanol (96 per cent).
IDENTIFICATION
First identification: B, E.
Second identification: A, C, D, E.
A. Melting point (2.2.14): 226 °C to 230 °C.
To 5 mL of solution S (see Tests) add 3 mL of dilute hydrochloric acid R. A white precipitate is formed. Filter and wash with water R. Dry the precipitate at 100-105 °C.
B. Infrared absorption spectrophotometry (2.2.24).
Preparation: Discs; dry the substances to constant mass at 105 °C before use.
Comparison: saccharin sodium CRS.
C. Mix about 10 mg with about 10 mg of resorcinol R, add 0.25 mL of sulfuric acid R and carefully heat the mixture over a naked flame until a dark green colour is produced. Allow to cool, add 10 mL of water R and dilute sodium hydroxide solution R until an alkaline reaction is produced. An intense green fluorescence develops.
D. To 0.2 g add 1.5 mL of dilute sodium hydroxide solution R, evaporate to dryness and heat the residue carefully until it melts, avoiding carbonisation. Allow to cool, dissolve the mass in about 5 mL of water R, add dilute hydrochloric acid R until a weak acid reaction is produced and filter, if necessary. To the filtrate add 0.2 mL of ferric chloride solution R2. A
violet colour develops.
E. Solution S gives reaction (a) of sodium (2.3.1).
TESTS
Solution S
Dissolve 5.0 g in carbon dioxide-free water R and dilute to 50.0 mL with the same solvent.
Appearance of solution
The solution is clear (2.2.1) and colourless (2.2.2, Method II).
Dissolve 2.0 g in 10 mL of water R.
Acidity or alkalinity
To 10 mL of solution S add about 0.05 mL of a 10 g/L solution of phenolphthalein R in ethanol (96 per cent) R. The solution is neither pink nor red. Add 0.05 mL of 0.1 M sodium hydroxide. The solution becomes pink or red.
o- and p-Toluenesulfonamide
Gas chromatography (2.2.28).
Internal standard solution: Dissolve 25 mg of caffeine R in methylene chloride R and dilute to 100 mL with the same solvent.
Test solution: Dissolve 10.0 g of the substance to be examined in 50 mL of water R. If necessary adjust the solution to pH 7-8 by addition of 1 M sodium hydroxide or 1 M hydrochloric acid. Shake the solution with 4 quantities, each of 50 mL, of methylene chloride R. Combine the lower layers, dry over anhydrous sodium sulfate R and filter. Wash the filter and the sodium sulfate with 10 mL of methylene chloride R. Combine the solution and the washings and evaporate almost to dryness in a water-bath at a temperature not exceeding 40 °C. Using a small quantity of methylene chloride R, quantitatively transfer the residue into a suitable 10 mL tube, evaporate to dryness in a current of nitrogen R and add 1.0 mL of the internal standard solution. Blank solution Evaporate 200 mL of methylene chloride R to dryness in a water-bath at a temperature not exceeding 40 °C. Dissolve the residue in 1 mL of methylene chloride R.
Reference solution: Dissolve 20.0 mg of o-toluenesulfonamide R and 20.0 mg of toluenesulfonamide R in methylene chloride R and dilute to 100.0 mL with the same solvent. Dilute 5.0 mL of the solution to 50.0 mL with methylene chloride R. Evaporate 5.0 mL of the final solution to dryness in a current of nitrogen R. Take up the residue using 1.0 mL of the internal standard solution.
Column:
— material: fused silica;
— size: l = 10 m, Ø = 0.53 mm;
— stationary phase: phenyl(50)methyl(50)polysiloxane R (film thickness 2 μm).
Carrier: gas nitrogen for chromatography R.
Flow rate: 10 mL/min.
Split ratio: 1:2.
Temperature:
— column: 180 °C;
— injection port and detector: 250 °C.
Detection: Flame ionisation.
Injection: 1 μL.
Elution order o-toluenesulfonamide, p-toluenesulfonamide, caffeine.
System suitability:
— resolution: minimum 1.5 between the peaks due to o-toluenesulfonamide and p-toluenesulfonamide in the chromatogram obtained with the reference solution;
— the chromatogram obtained with the blank solution does not show any peak with the same retention times as the internal standard, o-toluenesulfonamide and p-toluenesulfonamide.
Limits:
— o-toluenesulfonamide: the ratio of its area to that of the internal standard is not greater than the corresponding ratio in the chromatogram obtained with the reference solution (10 ppm);
— p-toluenesulfonamide: the ratio of its area to that of the internal standard is not greater than the corresponding ratio in the chromatogram obtained with the reference solution (10 ppm).
Readily carbonisable substances
Dissolve 0.200 g in 5 mL of sulfuric acid R and keep at 48-50 °C for 10 min. When viewed against a white background, the solution is not more intensely coloured than a solution prepared by mixing 0.1 mL of red primary solution, 0.1 mL of blue primary solution and 0.4 mL of yellow primary solution (2.2.2) with 4.4 mL of water R.
Water (2.5.12)
Maximum 15.0 per cent, determined on 0.200 g.
ASSAY
Dissolve 0.150 g in 50 mL of anhydrous acetic acid R, with slight heating if necessary. Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20). Carry out a blank titration.
1 mL of 0.1 M perchloric acid is equivalent to 20.52 mg of C7H4NNaO3S.
STORAGE
In an airtight container.