(Ph. Eur. monograph 1771)
C3H6O3 90.1
DEFINITION
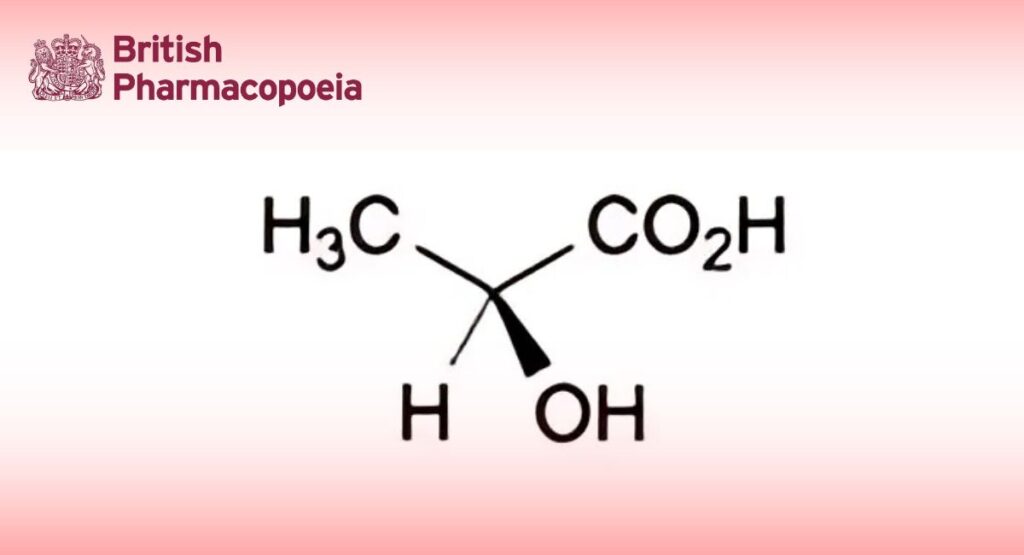
Mixture of (S)-2-hydroxypropanoic acid, its condensation products, such as lactoyl-lactic acid and polylactic acids, and water. The equilibrium between lactic acid and polylactic acids depends on the concentration and temperature.
Content
88.0 per cent m/m to 92.0 per cent m/m of C3H6O3, not less than 95.0 per cent of which is the (S)- enantiomer.
CHARACTERS
Appearance
Colourless or slightly yellow, syrupy liquid.
Solubility
Miscible with water and with ethanol (96 per cent).
IDENTIFICATION
A. Dissolve 1 g in 10 mL of water R. The solution is strongly acidic (2.2.4).
B. Relative density (2.2.5): 1.20 to 1.21.
C. It gives the reaction of lactates (2.3.1).
D. It complies with the limits of the assay.
TESTS
Solution S
Dissolve 5.0 g in 42 mL of 1 M sodium hydroxide and dilute to 50 mL with distilled water R.
Appearance
The substance to be examined is not more intensely coloured than reference solution Y6 (2.2.2, Method II).
Ether-insoluble substances
Dissolve 1.0 g in 25 mL of ether R. The solution is not more opalescent than the solvent used for the test.
Sugars and other reducing substances
To 1 mL of solution S add 1 mL of 1 M hydrochloric acid, heat to boiling, allow to cool and add 1.5 mL of 1 M sodium hydroxide and 2 mL of cupri-tartaric solution R. Heat to boiling. No red or greenish precipitate is formed.
Methanol (2.4.24)
Maximum 50 ppm, if intended for use in the manufacture of parenteral preparations.
Citric, oxalic and phosphoric acids
To 5 mL of solution S add dilute ammonia R1 until slightly alkaline (2.2.4). Add 1 mL of calcium chloride solution R. Heat on a water-bath for 5 min. Both before and after heating, any opalescence in the solution is not more intense than that in a mixture of 1 mL of water R and 5 mL of solution S.
Sulfates (2.4.13)
Maximum 200 ppm.
Dilute 7.5 mL of solution S to 15 mL with distilled water R.
Calcium (2.4.3)
Maximum 200 ppm.
Dilute 5 mL of solution S to 15 mL with distilled water R.
Sulfated ash (2.4.14)
Maximum 0.1 per cent, determined on 1.0 g.
Bacterial endotoxins (2.6.14)
Less than 5 IU/g if intended for use in the manufacture of parenteral preparations without a further appropriate procedure for the removal of bacterial endotoxins. Before use, neutralise the test solution to pH 7.0-7.5 with strong sodium hydroxide solution R and shake vigorously.
ASSAY
Place 1.000 g in a ground-glass-stoppered flask and add 10 mL of water R and 20.0 mL of 1 M sodium hydroxide. Close the flask and allow to stand for 30 min. Using 0.5 mL of phenolphthalein solution R as indicator, titrate with 1 M hydrochloric acid until the pink colour is discharged.
1 mL of 1 M sodium hydroxide is equivalent to 90.1 mg of C3H6O3.
(S)-enantiomer
Transfer an amount of the substance to be examined equivalent to 2.00 g of lactic acid into a round-bottomed flask, add 25 mL of 1 M sodium hydroxide and boil gently for 15 min. Cool down and adjust to pH 7.0 using 1 M hydrochloric acid. Add 5.0 g of ammonium molybdate R, dissolve and dilute to 50.0 mL with water R. Filter and measure the angle of optical rotation (2.2.7). Calculate the percentage content of (S)-enantiomer using the expression:
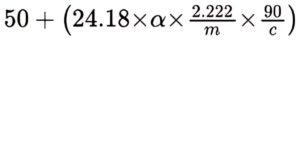
α = angle of optical rotation (absolute value),
m = mass of the substance to be examined, in grams,
c = percentage content of C3H6O3 in the substance to be examined.
The complex of (S)-lactic acid formed under these test conditions is laevorotatory.
LABELLING
The label states, where applicable, that the substance is suitable for use in the manufacture of parenteral preparations.