Action and use
Protease inhibitor; antiviral (HIV).
DEFINITION
Ritonavir Tablets contain Ritonavir.
The tablets comply with the requirements stated under Tablets and with the following requirements.
Content of ritonavir, C37H48N6O5S2
90.0 to 105.0% of the stated amount.
IDENTIFICATION
A. Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions prepared in a mixture of equal volumes of acetonitrile and water.
(1) Shake a quantity of the powdered tablets containing 20 mg of Ritonavir with 10 mL. Centrifuge and use the supernatant liquid.
(2) 0.2% w/v of ritonavir BPCRS.
CHROMATOGRAPHIC CONDITIONS
(a) Use as the coating silica gel F254 (Merck silica gel 60 F254 plates are suitable).
(b) Use the mobile phase as described below.
(c) Apply 2 μL of each solution.
(d) Develop the plate to 6 cm.
(e) After removal of the plate, dry in air and examine under ultraviolet light (254 nm).
MOBILE PHASE
5 volumes of glacial acetic acid, 6 volumes of n-heptane and 18 volumes of ethyl acetate.
CONFIRMATION
The principal spot in the chromatogram obtained with solution (1) corresponds in position and size to that in the chromatogram obtained with solution (2).
B. In the Assay, the principal peak in the chromatogram obtained with solution (1) corresponds to that in the chromatogram obtained with solution (2).
TESTS
Dissolution
Comply with the requirements in the dissolution test for tablets and capsules, Appendix XII B1.
TEST CONDITIONS
(a) Use Apparatus 2, rotating the paddle at 75 revolutions per minute.
(b) Use 900 mL of 0.06M polyoxyethylene 10 lauryl ether, at a temperature of 37°, as the medium.
PROCEDURE
Carry out the method for liquid chromatography, Appendix III D, using the following solutions
(1) After 120 minutes withdraw a sample of the medium and filter. Use the filtered medium, diluted with dissolution medium if necessary, expected to contain 0.011% w/v of Ritonavir.
(2) 0.11% w/v of ritonavir BPCRS in methanol R1. Dilute 1 volume to 10 volumes with dissolution medium.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (15 cm × 4.6 mm) packed with end-capped octadecylsilyl silica gel for chromatography (5 μm) (Nucleosil C18 is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1.5 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 215 nm.
(f) Inject 25 μL of each solution.
MOBILE PHASE
45 volumes of 0.03M potassium dihydrogen orthophosphate, adjusted to pH 4.0 using orthophosphoric acid, and 55 volumes of acetonitrile R1.
DETERMINATION OF CONTENT
Calculate the total content of ritonavir, C37H48N6O5S2, in the medium from the chromatograms obtained and using the declared content of C37H48N6O5S2, in ritonavir BPCRS.
LIMITS
The amount of ritonavir released is not less than 75% (Q) of the stated amount.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions. Pre-rinse all glassware with water.
Solution A: Equal volumes of acetonitrile and a 0.38% w/v solution of potassium dihydrogen orthophosphate.
Solution B: 10 volumes of a 0.38% w/v solution of potassium dihydrogen orthophosphate, 10 volumes of water, 15 volumes of butan-1-ol and 65 volumes of acetonitrile.
Solution C: 5 volumes of butan-1-ol, 15 volumes of acetonitrile and 80 volumes of a 0.38% w/v solution of potassium dihydrogen orthophosphate.
(1) Shake a quantity of powdered tablets containing 0.5 g of Ritonavir with 250 mL of solution B, shake and then mix with the aid of ultrasound. Add sufficient solution B to produce 500 mL and stir. Centrifuge and dilute 1 volume of the supernatant liquid to 2 volumes with solution C. Filter through a 0.2-μm nylon filter and use the filtrate.
(2) Dilute 1 volume of solution (1) to 100 volumes with solution C.
(3) Dilute 1 volume of solution (2) to 10 volumes with solution C.
(4) 0.1% w/v of ritonavir for peak identification EPCRS in solution A. Dilute 1 volume to 2 volumes with solution C.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (15 cm × 4.6 mm) packed with end-capped butylsilyl silica gel for chromatography (3 μm) (YMC C4 is suitable).
(b) Use gradient elution and the mobile phase described below.
(c) Use a flow rate of 1 mL per minute.
(d) Use a column temperature of 60°.
(e) Use a detection wavelength of 240 nm.
(f) Inject 50 μL of each solution.
MOBILE PHASE
Mobile phase A: 5 volumes of butan-1-ol, 8 volumes of tetrahydrofuran, stabiliser-free, 18 volumes of acetonitrile and 69 volumes of a solution containing 0.025% w/v of dipotassium hydrogen orthophosphate and 0.38% w/v of potassium dihydrogen orthophosphate. Adjust to pH 6.3 using 1Morthophosphoric acid or 1M potassium hydroxide.
Mobile phase B: 8 volumes of butan-1-ol, 13 volumes of tetrahydrofuran, stabiliser-free, 30 volumes of acetonitrile and 49 volumes of 0.38% w/v of potassium dihydrogen orthophosphate.
| Time (Minutes) | Mobile phase A (% v/v) | Mobile phase B (% v/v) | Comment |
| 0-80 | 100 | 0 | isocratic |
| 80-80.1 | 100→0 | 0→100 | linear gradient |
| 80.1-115 | 0 | 100 | isocratic column wash |
| 115-115.1 | 0→100 | 100→0 | linear gradient |
| 115.1-145 | 100 | 0 | re-equilibration |
When the chromatograms are recorded under the prescribed conditions the retention times relative to ritonavir (retention time about 31 minutes) are; impurity E, about 0.39; impurity F, about 0.41; impurity 1, about 0.7; impurity L, about 0.9 and
impurity O, about 1.1.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (4), the peak to valley ratio is at least 1.2, where Hp is the height above the baseline of the peak due to impurity E and Hv is the height above the baseline of the lowest point of the curve separating this peak from the peak due to impurity F.
LIMITS
Identify any peaks in the chromatogram obtained with solution (1) corresponding to impurities E, F, L and O using solution (4) and multiply the areas of these peaks by the corresponding correction factors: impurity F, 1.4 and impurity L, 1.9.
In the chromatogram obtained with solution (1):
the area of any peak corresponding to impurity F is not greater than 2.6 times the area of the principal peak in the chromatogram obtained with solution (2) (2.6%)
the area of any peaks corresponding to impurities E, O or L is not greater than 3 times the area of the principal peak in the chromatogram obtained with solution (3) (0.3% of each);
the area of any other secondary peak is not greater than twice the area of the principal peak in the chromatogram obtained with solution (3) (0.2%);
the sum of the area of any secondary peaks, excluding impurity F, is not greater than 0.9 times the area of the principal peak in the chromatogram obtained with solution (2) (0.9%).
Disregard any peak with an area less than half the area of the principal peak in the chromatogram obtained with solution (3) (0.05%).
ASSAY
Carry out the method for liquid chromatography, Appendix III D, using the following solutions. Pre-rinse all glassware with water.
Solution D: Equal volumes of acetonitrile and 0.03M potassium dihydrogen orthophosphate.
Solution E: 10 volumes of 0.03M potassium dihydrogen orthophosphate, 10 volumes of water, 15 volumes of butan-1-ol and 65 volumes of acetonitrile.
(1) To a number of whole tablets containing 1 g of Ritonavir, add 500 mL of solution E. Add sufficient solution E to produce 1000 mL and stir. Centrifuge and dilute 1 volume of the supernatant liquid to 10 volumes with solution D.
(2) 0.01% w/v solution of ritonavir BPCRS in solution D.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (15 cm × 4.6 mm) packed with base-deactivated octylsilyl silica gel for chromatography (5 μm) (Hypersil C8 is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1.5 mL per minute.
(d) Use a column temperature of 40°.
(e) Use a detection wavelength of 240 nm.
(f) Inject 50 μL of each solution.
MOBILE PHASE
10 volumes of methanol, 10 volumes of tetrahydrofuran, stabiliser-free, 17.5 volumes of acetonitrile and 62.5 volumes of 0.03M potassium dihydrogen orthophosphate.
DETERMINATION OF CONTENT
Calculate the content of ritonavir, C37H48N6O5S2, in the tablets from the chromatograms obtained and using the declared content of C37H48N6O5S2, in ritonavir BPCRS.
IMPURITIES
The impurities limited by the requirements of this monograph include impurities B to S listed under Ritonavir and:
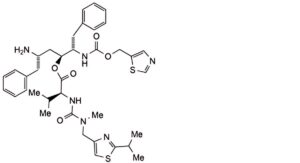
1. (1,3-thiazol-5-yl)methyl (5S,8S,9S)-8-[(2S)-2-amino-3-phenylpropyl]-9-benzyl-2-methyl-3,6-dioxo-5-(propan-2-yl)-1-[2-(propan-2-yl)-1,3-thiazol-4-yl]-7-oxa-2,4,10-triazaundecan-11-oate (geo isomer)






