Action and use
Protease inhibitor; antiviral (HIV).
DEFINITION
Ritonavir Oral Solution contains Ritonavir.
The oral solution complies with the requirements stated under Oral Liquids and with the following requirements.
Content of ritonavir, C37H48N6O5S2
92.0 to 105.0% of the stated amount.
IDENTIFICATION
A. Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions prepared in a mixture of equal volumes of acetonitrile and water.
(1) Dilute a volume of the oral solution to produce a solution containing 0.2% w/v of Ritonavir.
(2) 0.2% w/v of ritonavir BPCRS.
CHROMATOGRAPHIC CONDITIONS
(a) Use as the coating silica gel F254 (Merck silica gel 60 F254 plates are suitable).
(b) Use the mobile phase as described below.
(c) Apply 2 μL of each solution.
(d) Develop the plate to 6 cm.
(e) After removal of the plate, dry in air and examine under ultraviolet light (254 nm).
MOBILE PHASE
5 volumes of glacial acetic acid, 6 volumes of n-heptane and 18 volumes of ethyl acetate.
CONFIRMATION
The principal spot in the chromatogram obtained with solution (1) corresponds in position and size to that in the chromatogram obtained with solution (2).
B. In the Assay, the principal peak in the chromatogram obtained with solution (1) corresponds to that in the chromatogram obtained with solution (2).
TESTS
Acidity
pH, 3.7 to 4.7, Appendix V L.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions. Pre-rinse all glassware with water.
Solution A: Equal volumes of acetonitrile and a 0.38% w/v solution of potassium dihydrogen orthophosphate.
Solution B: 35 volumes of a 0.38% w/v solution of potassium dihydrogen orthophosphate and 65 volumes of acetonitrile.
Solution C: 8 volumes of butan-1-ol and 92 volumes of a 0.38% w/v solution of potassium dihydrogen orthophosphate.
(1) Dilute a quantity of oral solution containing 0.4 g of Ritonavir to 200 mL with solution B and dilute 1 volume of the resulting solution to 2 volumes with solution C.
To 3 volumes of this solution, add 4 volumes of n-heptane in a centrifuge
tube that has been previously rinsed with methanol and dried. Shake the tube vigorously until a uniform emulsion is obtained. Centrifuge the resulting emulsion and remove the clear top layer (heptane) and the viscous white cloudy middle layer, leaving the clear bottom layer in the tube. Repeat the procedure beginning at the words ‘add 4 volumes of n- heptane…’ and use the bottom layer.
(2) Dilute 1 volume of solution (1) to 100 volumes with solution C.
(3) Dilute 1 volume of solution (2) to 10 volumes with solution C.
(4) 0.1% w/v of ritonavir for peak identification EPCRS in solution A. Dilute 1 volume to 2 volumes with solution C.
(5) To a weighed quantity of oral solution containing 0.8 g of Ritonavir, add 1% w/w of citric acid and heat at 70° for 24 hours. Dilute 1 volume to 40 volumes with solution B. To 3 volumes of the resulting solution, add 4 volumes of n-heptane in a centrifuge tube that has been previously rinsed with methanol and dried. Shake the tube vigorously until a uniform emulsion is obtained. Centrifuge the resulting emulsion and remove the clear top layer (heptane) and the viscous white cloudy middle layer, leaving the clear bottom layer in the tube. Repeat the procedure beginning at the words ‘add 4 volumes of n-heptane…’ and use the bottom layer.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (15 cm × 4.6 mm) packed with end-capped butylsilyl silica gel for chromatography (3 μm) (YMC C4 is suitable).
(b) Use gradient elution and the mobile phase described below.
(c) Use a flow rate of 1 mL per minute.
(d) Use a column temperature of 60°.
(e) Use a detection wavelength of 240 nm.
(f) Inject 50 μL of each solution.
MOBILE PHASE
Mobile phase A: 5 volumes of butan-1-ol, 8 volumes of tetrahydrofuran, stabiliser-free, 18 volumes of acetonitrile and 69 volumes of a solution containing 0.025% w/v of dipotassium hydrogen orthophosphate and 0.38% w/v of potassium dihydrogen orthophosphate. Adjust to pH 6.3 using 1M orthophosphoric acid or 1M potassium hydroxide.
Mobile phase B: 8 volumes of butan-1-ol, 13 volumes of tetrahydrofuran, stabiliser-free, 30 volumes of acetonitrile and 49 volumes of 0.38% w/v of potassium dihydrogen orthophosphate.
| Time (Minutes) | Mobile phase A (% v/v) | Mobile phase B (% v/v) | Comment |
| 0-80 | 100 | 0 | isocratic |
| 80-80.1 | 100→0 | 0→100 | linear gradient |
| 80.1-115 | 0 | 100 | isocratic column wash |
| 115-115.1 | 0→100 | 100→0 | linear gradient |
| 115.1-145 | 100 | 0 | re-equilibration |
When the chromatograms are recorded under the prescribed conditions the retention times relative to ritonavir (retention time about 34 minutes) are; impurity B, about 0.1; impurity 3, about 0.25; impurity E, about 0.35; impurity F, about 0.4; impurity 2, about 0.45; impurity 1, about 0.7; impurity L, about 0.9 and impurity O, about 1.1.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (4), the peak to valley ratio is at least 1.2, where Hp is the height above the baseline of the peak due to impurity E and Hv is the height above the baseline of the lowest point of the curve separating this peak from the peak due to impurity F.
LIMITS
Identify any peaks in the chromatogram obtained with solution (1) corresponding to impurities E, F, L and O using solution (4) and impurities 2 and 3 using solution (5) and multiply the areas of these peaks by the corresponding correction factors: impurity 2, 1.5; impurity 3, 1.7, impurity F, 1.4 and impurity L, 1.9.
In the chromatogram obtained with solution (1):
the area of any peak corresponding to impurity 2 is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (1.0%);
the area of any peak corresponding to impurity B or impurity E is not greater than half the area of the principal peak in the chromatogram obtained with solution (2) (0.5% of each);
the area of any peak corresponding to impurity 3 or impurity F is not greater than 0.4 times the area of the principal peak in the chromatogram obtained with solution (2) (0.4% of each);
the area of any peak corresponding to impurity O is not greater than 3 times the area of the principal peak in the chromatogram obtained with solution (3) (0.3%);
the area of any other secondary peak is not greater than twice the area of the principal peak in the chromatogram obtained with solution (3) (0.2%);
the sum of the area of any secondary peaks is not greater three times the area of the principal peak in the chromatogram obtained with solution (2) (3.0%).
Disregard any peak with an area less than half the area of the principal peak in the chromatogram obtained with solution (3) (0.05%).
ASSAY
Carry out the method for liquid chromatography, Appendix III D, using the following solutions. Pre-rinse all glassware with water.
Solution D: Equal volumes of acetonitrile and 0.03M potassium dihydrogen orthophosphate.
(1) Mix a weighed quantity of the oral solution containing 0.25 g of Ritonavir with 80 mL of solution D with the aid of ultrasound, and dilute to 100 mL. Dilute 1 volume to 100 volumes with solution D.
(2) 0.0025% w/v solution of ritonavir BPCRS in solution D.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (15 cm × 4.6 mm) packed with base-deactivated octylsilyl silica gel for chromatography (5 μm) (Hypersil C8 is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 1.5 mL per minute.
(d) Use a column temperature of 40°.
(e) Use a detection wavelength of 240 nm.
(f) Inject 50 μL of each solution.
MOBILE PHASE
10 volumes of methanol, 10 volumes of tetrahydrofuran, stabiliser-free, 17.5 volumes of acetonitrile and 62.5 volumes of 0.03M potassium dihydrogen orthophosphate.
DETERMINATION OF CONTENT
Determine the weight per mL of the Oral Solution, Appendix V G, and calculate the content of ritonavir, C37H48N6O5S2 using the declared content of C37H48N6O5S2 in ritonavir BPCRS.
IMPURITIES
The impurities limited by the requirements of this monograph include impurities B to S listed under Ritonavir and the following:
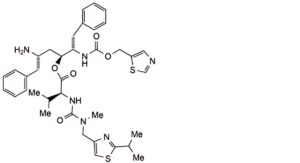
1. (1,3-thiazol-5-yl)methyl (5S,8S,9S)-8-[(2S)-2-amino-3-phenylpropyl]-9-benzyl-2-methyl-3,6-dioxo-5-(propan-2-yl)-1-[2-(propan-2-yl)-1,3-thiazol-4-yl]-7-oxa-2,4,10-triazaundecan-11-oate (geo isomer)
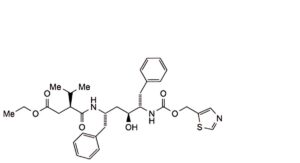
2. (1,3-thiazol-5-yl)methyl (3S,4S,6S,9S)-3,6-dibenzyl-4-hydroxy-8,11-dioxo-9-(propan-2-yl)-12-oxa-2,7,10-triazatetradecanoate (ethanol adduct)
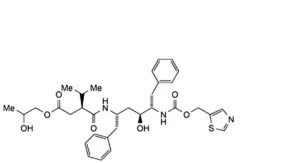
3. (1,3-thiazol-5-yl)methyl (3S,4S,6S,9S,14RS)-3,6-dibenzyl-4,14-dihydroxy-8,11-dioxo-9-(propan-2-yl)-12-oxa-2,7,10-triazapentadecanoate (propylene glycol adduct)






