Dopamine D2 receptor antagonist; serotonin 5HT2 receptor antagonist; neuroleptic.
DEFINITION
Risperidone Oral Solution is a solution of Risperidone in a suitable vehicle.
The oral solution complies with the requirements stated under Oral Liquids and with the following requirements.
Content of risperidone, C23H27FN4O2
95.0 to 105.0% of the stated amount.
IDENTIFICATION
A. Carry out the method for thin-layer chromatography, Appendix III A, using the following solutions in methanol.
(1) Dilute a volume of the oral solution to contain 0.01% w/v of Risperidone.
(2) 0.01% w/v of risperidone BPCRS.
(3) 0.01% w/v each of risperidone BPCRS and trazodone hydrochloride BPCRS.
CHROMATOGRAPHIC CONDITIONS
(a) Use as the coating silica gel F254.
(b) Use the mobile phase as described below.
(c) Apply 20 μL of each solution.
(d) Develop the plate to 15 cm.
(e) After removal of the plate, dry in a current of air and examine under ultraviolet light (254 nm).
MOBILE PHASE
3 volumes of glacial acetic acid, 5 volumes of water and 12 volumes of butan-1-ol.
SYSTEM SUITABILITY
The test is not valid unless the chromatogram obtained with solution (3) shows two clearly separated spots.
CONFIRMATION
The principal spot in the chromatogram obtained with solution (1) corresponds in position and colour to that in the chromatogram obtained with solution (2).
B. In the Assay, the principal peak in the chromatogram obtained with solution (1) has the same retention time as the principal peak in the chromatogram obtained with solution (2).
TESTS
Acidity
pH, 2.0 to 4.0, Appendix V L.
Related substances
Carry out the method for liquid chromatography, Appendix III D, using the following solutions prepared immediately before use.
(1) Dilute a quantity of the oral solution with 0.1M hydrochloric acid, if necessary, to contain 0.01% w/v of Risperidone.
(2) Dilute 1 volume of solution (1) to 200 volumes with 0.1M hydrochloric acid.
(3) Dilute 1 volume of solution (2) to 5 volumes with 0.1M hydrochloric acid.
(4) 0.005% w/v of risperidone impurity standard BPCRS in 0.1M hydrochloric acid.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (15 cm × 4.6 mm) packed with base-deactivated octadecylsilyl silica gel for chromatography (5 μm) (Zorbax SB-C18 is suitable).
(b) Use gradient elution and the mobile phase described below.
(c) Use a flow rate of 2.5 mL per minute. Equilibrate the column for at least 30 minutes with methanol at the end of each run.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 275 nm.
(f) Inject 50 μL of each solution.
MOBILE PHASE
Mobile phase A: 0.1 volumes of trifluoroacetic acid, 20 volumes of acetonitrile and 80 volumes of water, adjusting the pH of the mixture to 3.0 with 13.5M ammonia.
Mobile phase B: 0.1 volumes of trifluoroacetic acid, 39 volumes of methanol and 61 volumes of water, adjusting the pH to 3.0 with 13.5M ammonia.
| Time (Minutes) | Mobile phase A (% v/v) | Mobile phase B (% v/v) | Comment |
| 0-8 | 100 | 0 | isocratic |
| 8-16 | 100→0 | 0→100 | linear gradient |
| 16-20 | 0 | 100 | isocratic |
| 20-21 | 0→100 | 100→0 | linear gradient |
| 21-30 | 100 | 0 | re-equilibration |
When the chromatograms are recorded under the prescribed conditions the retention times relative to risperidone (retention time about 11 minutes) are: impurity B, about 0.6; impurity 2, about 0.7 and impurity 1, about 1.8.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (4), the resolution between impurity B and impurity 2 is at least 1.5.
LIMITS
In the chromatogram obtained with solution (1):
the area of any peak corresponding to impurity 1 is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.5%);
the area of any peak corresponding to impurity 2 is not greater than the area of the principal peak in the chromatogram obtained with solution (2) (0.5%);
the area of any other secondary peak is not greater than twice the area of the principal peak in the chromatogram obtained with solution (3) (0.2%);
the sum of the areas of any secondary peaks is not greater than twice the area of the principal peak in the chromatogram obtained with solution (2) (1.0%).
Disregard any peak with an area less than the area of the principal peak in the chromatogram obtained with solution (3) (0.1%).
ASSAY
Carry out the method for liquid chromatography, Appendix III D, using the following solutions.
(1) Dilute a quantity of the oral solution with 0.1M hydrochloric acid, if necessary, to contain 0.01% w/v of Risperidone and filter through a 0.45-μm filter.
(2) 0.01% w/v of risperidone BPCRS in 0.1M hydrochloric acid.
(3) 0.01% w/v of risperidone impurity standard BPCRS in 0.1M hydrochloric acid.
CHROMATOGRAPHIC CONDITIONS
(a) Use a stainless steel column (15 cm × 4.6 mm) packed with base-deactivated octadecylsilyl silica gel for chromatography (5 μm) (Zorbax SB-C18 is suitable).
(b) Use isocratic elution and the mobile phase described below.
(c) Use a flow rate of 2.5 mL per minute.
(d) Use an ambient column temperature.
(e) Use a detection wavelength of 237 nm.
(f) Inject 50 μL of each solution.
MOBILE PHASE
0.1 volumes of trifluoroacetic acid, 20 volumes of acetonitrile and 80 volumes of water adjusting the pH of the mixture to 3.0 with 13.5M ammonia.
When the chromatograms are recorded under the prescribed conditions the retention times relative to risperidone (retention time about 10 minutes) are: impurity B, about 0.65, impurity 2, about 0.7 and impurity 1, about 1.6.
SYSTEM SUITABILITY
The test is not valid unless, in the chromatogram obtained with solution (3), the resolution between impurity 2 and impurity B is at least 1.5.
DETERMINATION OF CONTENT
Calculate the total content of risperidone, C23H27FN4O2, in the medium from the chromatograms obtained and using the declared content of C23H27FN4O2 in risperidone BPCRS.
IMPURITIES
The impurities limited by the requirements of this monograph include impurity B, listed under Risperidone and the following:
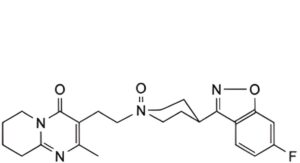
1. cis-4-(6-fluoro-1,2-benzoxazol-3-yl)-1-[2-(2-methyl-4-oxo-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-3- yl)ethyl]piperidine N-oxide hydrate
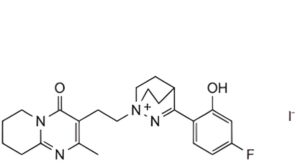
2. 3-(4-fluoro-2-hydroxyphenyl)-1-[2-(2-methyl-4-oxo-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-3-yl)ethyl]-1,2- diazabicyclo[2.2.2]oct-2-en-1-ium iodide






