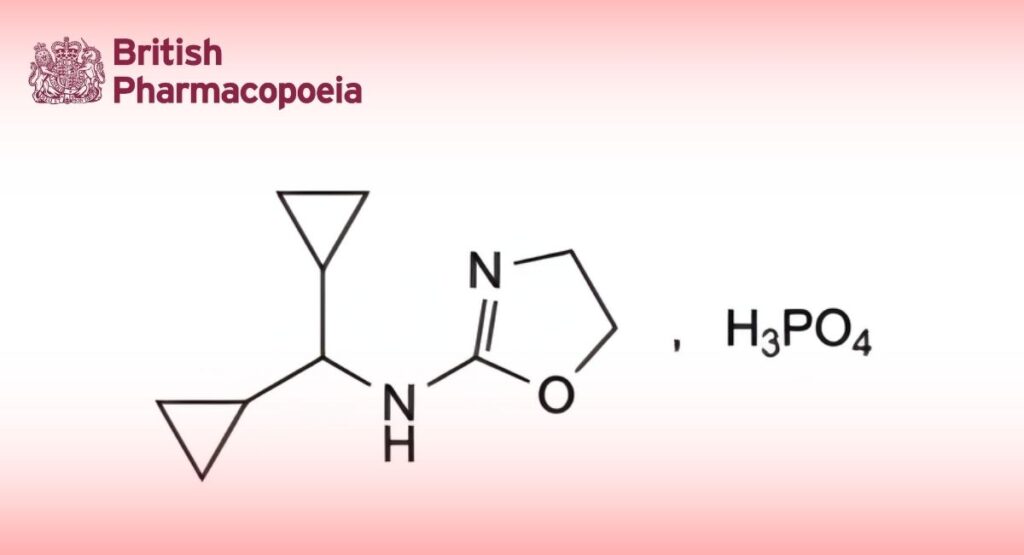
(Ph. Eur. monograph 2020)
C10H19N2O5P 278.2 85409-38-7
Action and use
Antihypertensive.
DEFINITION
N-(Dicyclopropylmethyl)-4,5-dihydro-oxazol-2-amine dihydrogen phosphate.
Content
99.0 per cent to 101.0 per cent (dried substance).
CHARACTERS
Appearance
White or almost white powder.
Solubility
Freely soluble in water, slightly soluble in ethanol (96 per cent), practically insoluble in methylene chloride.
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24).
Comparison: Ph. Eur. reference spectrum of rilmenidine dihydrogen phosphate.
B. Dissolve 10 mg in water R and dilute to 1 mL with the same solvent. The solution gives reaction (b) of phosphates (2.3.1).
TESTS
Related substances
Liquid chromatography (2.2.29).
Test solution: Dissolve 60.0 mg of the substance to be examined in water R and dilute to 20.0 mL with the same solvent.
Reference solution (a): Dilute 1.0 mL of the test solution to 100.0 mL with water R and dilute 10.0 mL of this solution to 50.0 mL with the same solvent.
Reference solution (b): Dilute 5.0 mL of reference solution (a) to 20.0 mL with water R.
Reference solution (c): Dissolve 15.0 mg of rilmenidine for system suitability CRS in water R and dilute to 5.0 mL with the same solvent.
Column:
— size: l = 0.15 m, Ø = 3 mm,
— stationary phase: base-deactivated octadecylsilyl silica gel for chromatography R (5 μm) with a pore size of 10 nm and a carbon loading of 25 per cent,
— temperature: 40 °C.
Mobile phase:
— mobile phase A: dissolve 3 g of sodium heptanesulfonate R in water R and dilute to 860 mL with the same solvent; add 130 mL of methanol R2, 10 mL of tetrahydrofuran for chromatography R and 1.0 mL of phosphoric acid R,
— mobile phase B: dissolve 3 g of sodium heptanesulfonate R in water R and dilute to 600 mL with the same solvent; add 350 mL of acetonitrile for chromatography R, 50 mL of tetrahydrofuran for chromatography R and 1.0 mL of phosphoric acid R,
| Time (min) |
Mobile phase A (per cent V/V) |
Mobile phase B (per cent V/V) |
| 0 – 14 | 100 → 0 | 0 → 100 |
| 14 – 15 | 0 → 100 | 100 → 0 |
| 15 – 30 | 100 | 0 |
Flow rate: 1 mL/min.
Detection: Spectrophotometer at 205 nm.
Injection: 20 μL.
Relative retention: With reference to rilmenidine (retention time = about 13 min): impurity A = about 0.6; impurity B = about 0.9; impurity C = about 1.4.
With these conditions the inflexion of the baseline, corresponding to the beginning of the gradient, appears on the recorder after a minimum time t of 5 min. If this is not the case (t < 5 min) modify the chromatographic sequence by adding an isocratic elution with 100 per cent of mobile phase A for a time corresponding to (5-t) min before the linear gradient.
System suitability: Reference solution (c):
— peak-to-valley ratio: minimum 3, where Hp = height above the baseline of the peak due to impurity B and Hv = height above the baseline of the lowest point of the curve separating this peak from the peak due to rilmenidine.
Limits:
— any impurity: not more than 0.5 times the area of the principal peak in the chromatogram obtained with reference solution (a) (0.1 per cent),
— total: not more than the area of the principal peak in the chromatogram obtained with reference solution (a) (0.2 per cent),
— disregard limit: area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 per cent).
Loss on drying (2.2.32)
Maximum 0.5 per cent, determined on 1.000 g by drying in vacuo at 50 °C for 2 h.
ASSAY
Dissolve 0.200 g in 50 mL of anhydrous acetic acid R. Titrate with 0.1 M perchloric acid, determining the end-point potentiometrically (2.2.20).
1 mL of 0.1 M perchloric acid is equivalent to 27.82 mg of C10H19N2O5P.
IMPURITIES
Specified impurities A, B, C.
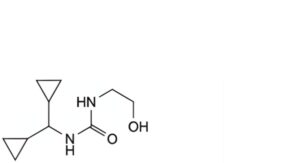
A. 1-(dicyclopropylmethyl)-3-(2-hydroxyethyl)urea,
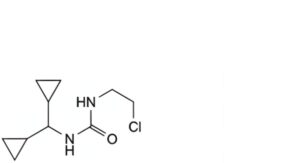
B. 1-(2-chloroethyl)-3-(dicyclopropylmethyl)urea,
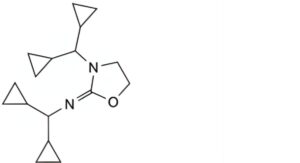
C. N,3-bis(dicyclopropylmethyl)oxazolidin-2-imine.