Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Rifamycin antimycobacterial drug.
DEFINITION
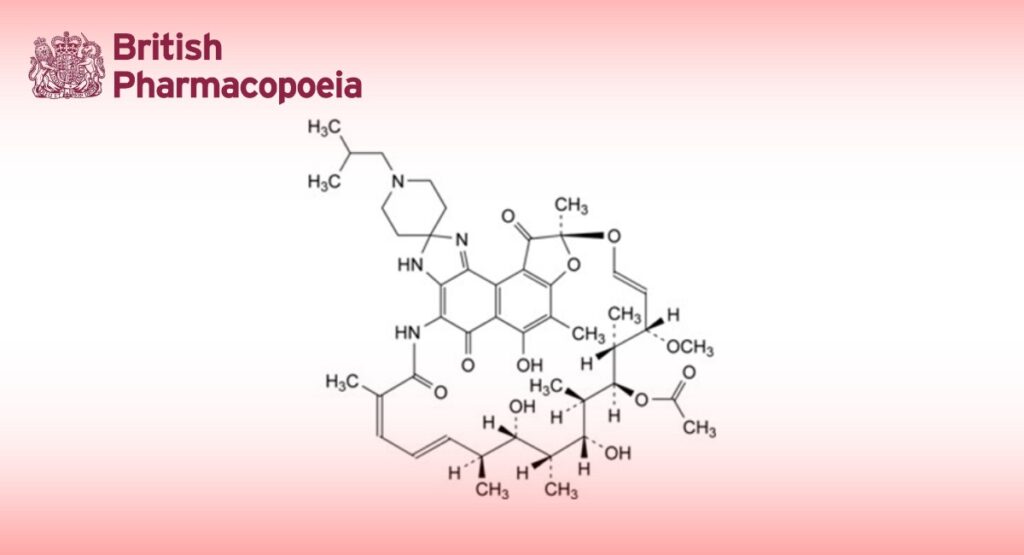
(9S,12E,14S,15R,16S,17R,18R,19R,20S,21S,22E,24Z)-6,18,20-Trihydroxy-14-methoxy-7,9,15,17,19,21,25-heptamethyl-1′-(2-methylpropyl)-5,10,26-trioxo-3,5,9,10-tetrahydrospiro[9,4-(epoxypentadeca[1,11,13]trienimino)-2H- furo[2′,3′:7,8]naphtho[1,2-d]imidazole-2,4′-piperidine]-16-yl acetate.
Semi-synthetic product derived from a fermentation product.
Content
96.0 per cent to 102.0 per cent (anhydrous substance).
CHARACTERS
Appearance
Reddish-violet amorphous powder.
Solubility
Slightly soluble in water, soluble in methanol, slightly soluble in ethanol (96 per cent).
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24).
Preparation Discs.
Comparison rifabutin CRS.
B. Examine the chromatograms obtained in the test for related substances.
Results The principal peak in the chromatogram obtained with the test solution is similar in retention time and size to the principal peak in the chromatogram obtained with reference solution (a).
TESTS
Impurity A
Thin-layer chromatography (2.2.27).
Test solution Dissolve 0.100 g of the substance to be examined in a mixture of equal volumes of methanol R and methylene chloride R and dilute to 10 mL with the same mixture of solvents.
Reference solution Dissolve 10 mg of rifabutin impurity A CRS in a mixture of equal volumes of methanol R and methylene chloride R and dilute to 10 mL with the same mixture of solvents. Dilute 3 mL of the solution to 100 mL with a mixture of equal volumes of methanol R and methylene chloride R.
Plate TLC silica gel F254 plate R.
Mobile phase acetone R, light petroleum R (23:77 V/V). Application 10 µL.
Development Over 2/3 of the plate.
Drying In air.
Detection Expose the plate to iodine vapour for about 5 min, then spray with potassium iodide and starch solution R and allow to stand for 5 min.
Limit:
— impurity A: any spot corresponding to impurity A is not more intense than the spot in the chromatogram obtained with the reference solution (0.3 per cent).
Related substances
Liquid chromatography (2.2.29).
Test solution Dissolve 50.0 mg of the substance to be examined in the mobile phase and dilute to 50.0 mL with the mobile phase.
Reference solution (a) Dissolve 50.0 mg of rifabutin CRS in the mobile phase and dilute to 50.0 mL with the mobile phase.
Reference solution (b) Dilute 1.0 mL of reference solution (a) to 100.0 mL with the mobile phase.
Reference solution (c) Dissolve about 10 mg of rifabutin CRS in 2 mL of methanol R, add 1 mL of dilute sodium hydroxide solution R and allow to stand for about 4 min. Add 1 mL of dilute hydrochloric acid R and dilute to 50 mL with the mobile phase.
Column:
— size: l = 0.110 m, Ø = 4.6 mm,
— stationary phase: octylsilyl silica gel for chromatography R (5 µm).
Mobile phase Mix equal volumes of acetonitrile R and a 13.6 g/L solution of potassium dihydrogen phosphate R adjusted to pH 6.5 with dilute sodium hydroxide solution R.
Flow rate 1 mL/min.
Detection Spectrophotometer at 254 nm.
Injection 20 µL.
Run time 2.5 times the retention time of rifabutin.
Relative retention With reference to rifabutin (retention time = about 9 min): impurity E = about 0.5; impurity B = about 0.6; impurity D = about 0.9; impurity C = about 1.3.
System suitability Reference solution (c):
— resolution: minimum 2.0 between the second peak of the 3 peaks due to degradation products and the peak due to rifabutin.
Limits:
— any impurity: not more than the area of the principal peak in the chromatogram obtained with reference solution (b) (1.0 per cent); not more than 1 such peak has an area greater than half the area of the principal peak in the chromatogram obtained with reference solution (b) (0.5 per cent),
— total: not more than 3 times the area of the principal peak in the chromatogram obtained with reference solution (b) (3.0 per cent),
— disregard limit: 0.05 times the area of the principal peak in the chromatogram obtained with reference solution (b) (0.05 per cent).
Water (2.5.12)
Maximum 2.5 per cent, determined on 0.200 g.
Sulfated ash (2.4.14)
Maximum 0.3 per cent, determined on 1.0 g.
ASSAY
Liquid chromatography (2.2.29) as described in the test for related substances with the following modification.
Injection Test solution and reference solution (a). Calculate the percentage content of ribabutin.
IMPURITIES
A. 1-(2-methylpropyl)piperidin-4-one,
B. 3-aminorifamycin S,
C. 21,31-didehydrorifabutin,
D. 3-amino-4-imidorifamycin S,
E. 16-deacetylrifabutin.