Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Vitamin B2.
Preparation
Vitamins B and C Injection Ph Eur
DEFINITION
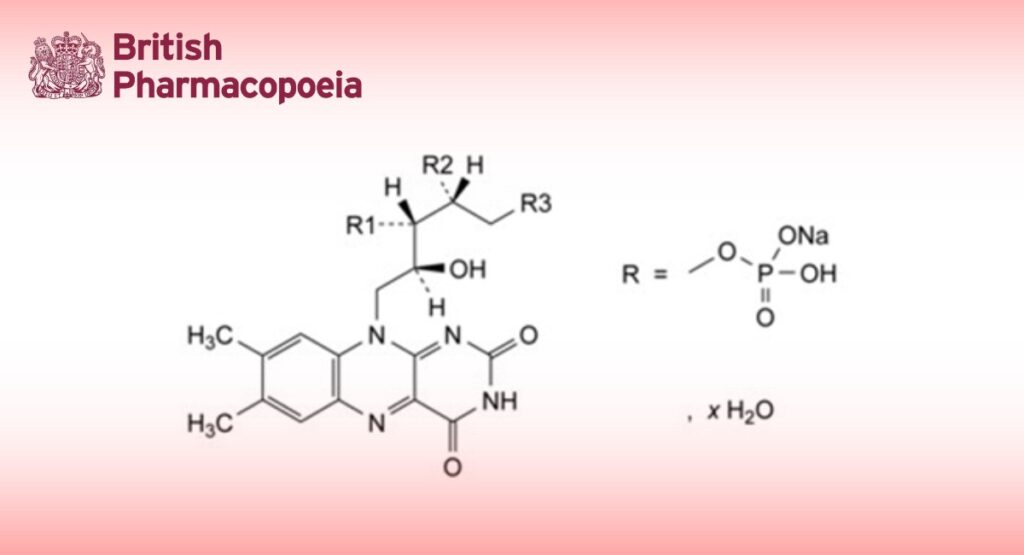
Semi-synthetic product derived from phosphorylation of Riboflavin (0292).
Main component:
— riboflavin 5′-(sodium hydrogen phosphate): sodium (2R,3S,4S)-5-(7,8-dimethyl-2,4-dioxo-3,4- dihydrobenzo[g]pteridin-10(2H)-yl)-2,3,4-trihydroxypentyl hydrogen phosphate.
Minor components:
— riboflavin: 7,8-dimethyl-10-[(2S,3S,4R)-2,3,4,5-tetrahydroxypentyl]benzo[g]pteridin-2,4(3H,10H)-dione;
— riboflavin 3′-(sodium hydrogen phosphate): sodium (2S,3S,4R)-1-(7,8-dimethyl-2,4-dioxo-3,4- dihydrobenzo[g]pteridin-10(2H)-yl)-2,4,5-trihydroxypentan-3-yl hydrogen phosphate;
— riboflavin 4′-(sodium hydrogen phosphate): sodium (2R,3S,4S)-5-(7,8-dimethyl-2,4-dioxo-3,4- dihydrobenzo[g]pteridin-10(2H)-yl)-1,3,4-trihydroxypentan-2-yl hydrogen phosphate;
— riboflavin 3′,4′-bis(sodium hydrogen phosphate): disodium (2R,3S,4S)-5-(7,8-dimethyl-2,4-dioxo-3,4- dihydrobenzo[g]pteridin-10(2H)-yl)-1,4-dihydroxypentane-2,3-diyl bis(hydrogen phosphate);
— riboflavin 3′,5′-bis(sodium hydrogen phosphate): disodium (2R,3S,4S)-5-(7,8-dimethyl-2,4-dioxo-3,4- dihydrobenzo[g]pteridin-10(2H)-yl)-2,4-dihydroxypentane-1,3-diyl bis(hydrogen phosphate);
— riboflavin 4′,5′-bis(sodium hydrogen phosphate): disodium (2R,3S,4S)-5-(7,8-dimethyl-2,4-dioxo-3,4- dihydrobenzo[g]pteridin-10(2H)-yl)-3,4-dihydroxypentane-1,2-diyl bis(hydrogen phosphate).
It contains a variable quantity of water.
Content
73.0 per cent to 79.0 per cent of riboflavin (C17H20N4O6; Mr 376.4) (dried substance).
CHARACTERS
Appearance
Yellow or orange-yellow, crystalline, hygroscopic powder.
Solubility
Soluble in water, very slightly soluble in ethanol (96 per cent).
IDENTIFICATION
A. Infrared absorption spectrophotometry (2.2.24).
Comparison riboflavin sodium phosphate CRS.
B. To 0.5 g add 10 mL of nitric acid R and evaporate the mixture to dryness. Ignite the residue until it becomes white, dissolve the residue in 5 mL of water R and filter.
The filtrate gives reaction (a) of sodium (2.3.1).
C. The filtrate obtained in identification test B gives reaction (b) of phosphates (2.3.1).
TESTS
pH (2.2.3)
5.0 to 6.5.
Dissolve 0.5 g in carbon dioxide-free water R and dilute to 50 mL with the same solvent.
Specific optical rotation (2.2.7)
+ 38.0 to + 43.0 (dried substance).
Dissolve 0.300 g in 18.2 mL of hydrochloric acid R1 and dilute to 25.0 mL with water R.
Impurity E
To about 35 mg add 10 mL of methylene chloride R, shake for 5 min and filter. The filtrate is not more intensely coloured than reference solution BY6 (2.2.2, Method II).
Related substances
Liquid chromatography (2.2.29): use the normalisation procedure. Carry out the test protected from light.
Test solution Dissolve 0.100 g of the substance to be examined in 50 mL of water R and dilute to 100.0 mL with the mobile phase. Dilute 8.0 mL of this solution to 50.0 mL with the mobile phase.
Reference solution (a) Dissolve 10 mg of riboflavin sodium phosphate CRS in 5 mL of water R and dilute to 10 mL with the mobile phase. Dilute 8 mL of this solution to 50 mL with the mobile phase.
Reference solution (b) Dilute 1.0 mL of the test solution to 100.0 mL with the mobile phase. Dilute 1.0 mL of this solution to 20.0 mL with the mobile phase.
Reference solution (c) Dissolve 5 mg of riboflavin sodium phosphate for peak identification CRS (containing impurities F, G and H) in 2.5 mL of water R and dilute to 5 mL with the mobile phase. Dilute 4 mL of the solution to 25 mL with the mobile phase.
Column:
— size: l = 0.25 m, Ø = 4.6 mm;
— stationary phase: base-deactivated end-capped octadecylsilyl silica gel for chromatography R (5 µm).
Mobile phase methanol R, 7.35 g/L solution of potassium dihydrogen phosphate R (15:85 V/V). Flow rate 2 mL/min.
Detection Spectrophotometer at 266 nm.
Injection 100 µL.
Run time 2.5 times the retention time of riboflavin 5′-monophosphate.
Identification of impurities use the chromatogram supplied with riboflavin sodium phosphate for peak identification CRS and the chromatogram obtained with reference solution (c) to identify the peaks due to impurities F, G and H.
Relative retention With reference to riboflavin 5′-monophosphate (retention time = about 17 min): riboflavin 3′, 4′- diphosphate = about 0.2; riboflavin 3′, 5′-diphosphate = about 0.3; riboflavin 4′, 5′-diphosphate = about 0.5; impurity F = about 0.6; riboflavin 3′-monophosphate = about 0.7; impurity G = about 0.8; riboflavin 4′-monophosphate = about 0.9; impurity H = about 1.3; riboflavin = about 2.
System suitability Reference solution (a):
— resolution: minimum 1.5 between the peaks due to riboflavin 4′-monophosphate and riboflavin 5′-monophosphate.
Limits:
— impurity F: maximum 0.7 per cent;
— impurity G: maximum 0.6 per cent;
— impurity H: maximum 0.4 per cent;
— unspecified impurities: for each impurity, maximum 0.2 per cent;
— total: maximum 2.0 per cent;
— reporting threshold: 0.05 per cent (reference solution (b)).
The thresholds indicated under Related substances (Table 2034.-1) in the general monograph Substances for pharmaceutical use (2034) do not apply.
Composition
Liquid chromatography (2.2.29) as described in the test for related substances.
Identification of peaks Use the chromatogram supplied with riboflavin sodium phosphate CRS and the chromatogram obtained with reference solution (a) to identify the peaks due to the major and minor components listed under Definition.
Limits:
— riboflavin 5′-(sodium hydrogen phosphate) hydrate: minimum 65.0 per cent;
— riboflavin 4′-(sodium hydrogen phosphate) hydrate: maximum 12.0 per cent;
— riboflavin 3′-(sodium hydrogen phosphate) hydrate: maximum 7.0 per cent;
— riboflavin hydrate: maximum 6.0 per cent;
— riboflavin 3′,5′-bis(sodium hydrogen phosphate) hydrate: maximum 3.5 per cent;
— riboflavin 4′,5′-bis(sodium hydrogen phosphate) hydrate: maximum 3.5 per cent;
— riboflavin 3′,4′-bis(sodium hydrogen phosphate) hydrate: maximum 1.0 per cent.
Inorganic phosphate
Maximum 1.0 per cent.
Acid molybdate solution Dilute 25 mL of a 70 g/L solution of ammonium molybdate R to 200 mL with water R. To this solution slowly add 25 mL of a 210 mL/L solution of sulfuric acid R.
Ferrous sulfate solution A 100 g/L solution of ferrous sulfate R in a 4.2 mL/L solution of sulfuric acid R.
Test solution Dissolve 0.150 g in water R and dilute to 50 mL with the same solvent. To 10.0 mL of the solution add 10.0 mL of acid molybdate solution and 5.0 mL of ferrous sulfate solution and mix.
Reference solution Immediately before use, dilute with water R to 10 times its volume a solution containing potassium dihydrogen phosphate R equivalent to 0.440 g of KH2PO4 in 1000.0 mL. To 10.0 mL of this solution add 10.0 mL of acid molybdate solution and 5.0 mL of ferrous sulfate solution and mix.
Measure the absorbance (2.2.25) of the test solution and the reference solution at 700 nm using water R as the compensation liquid.
Results The absorbance of the test solution is not greater than that of the reference solution.
Loss on drying (2.2.32)
Maximum 8.0 per cent, determined on 1.000 g by drying in vacuo at 105 °C for 5 h.
ASSAY
Carry out the assay protected from light.
Dissolve 0.100 g in 150 mL of water R, add 2 mL of glacial acetic acid R and dilute to 1000.0 mL with water R. To 10.0 mL of this solution add 3.5 mL of a 14 g/L solution of sodium acetate R and dilute to 50.0 mL with water R. Measure the absorbance (2.2.25) at the absorption maximum at 444 nm.
Calculate the content of C17H20N4O6 taking the specific absorbance to be 328.
STORAGE
In an airtight container, protected from light.
IMPURITIES
Specified impurities E, F, G, H.
E. 7,8,10-trimethylbenzo[g]pteridine-2,4(3H,10H)-dione (lumiflavin),
F. unknown structure,
G. unknown structure,
H. unknown structure.