Edition: BP 2025 (Ph. Eur. 11.6 update)
Action and use
Keratolytic.
DEFINITION
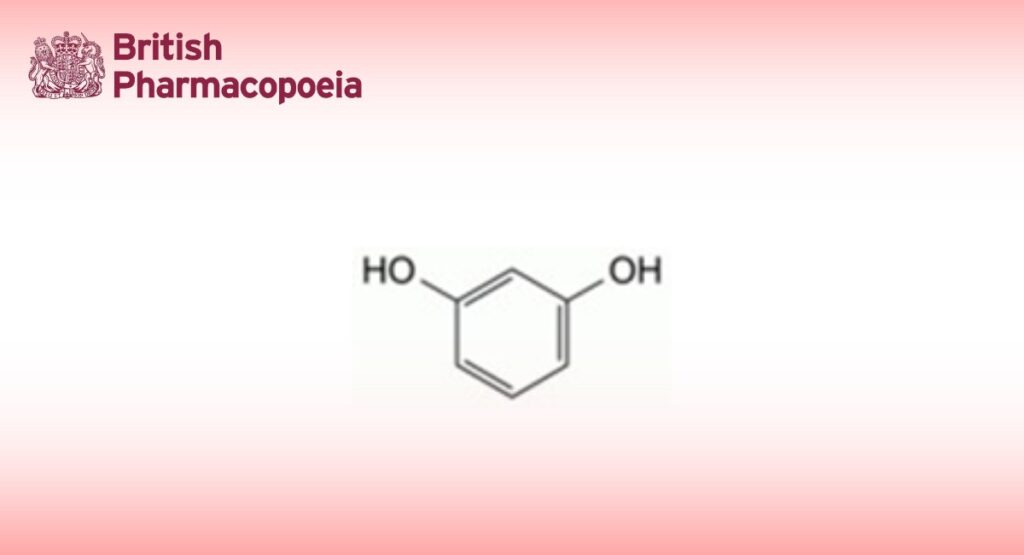
Resorcinol contains not less than 98.5 per cent and not more than the equivalent of 101.0 per cent of benzene-1,3-diol, calculated with reference to the dried substance.
CHARACTERS
A colourless or slightly pinkish-grey, crystalline powder or crystals, turning red on exposure to light and air, very soluble in water and in ethanol (96 per cent).
IDENTIFICATION
A. Melting point (2.2.14): 109 °C to 112 °C.
B. Dissolve 0.1 g in 1 mL of water R, add 1 mL of strong sodium hydroxide solution R and 0.1 mL of chloroform R, heat and allow to cool. An intense, deep-red colour develops which becomes pale yellow on the addition of a slight excess of hydrochloric acid R.
C. Thoroughly mix about 10 mg with about 10 mg of potassium hydrogen phthalate R, both finely powdered. Heat over a naked flame until an orange-yellow colour is obtained. Cool and add 1 mL of dilute sodium hydroxide solution R and 10 mL of water R and shake to dissolve. The solution shows an intense green fluorescence.
TESTS
Solution S
Dissolve 2.5 g in carbon dioxide-free water R and dilute to 25 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and not more intensely coloured than reference solution B5 or R5 (2.2.2, Method II) and remains so when heated in a water-bath for 5 min.
Acidity or alkalinity
To 10 mL of solution S add 0.05 mL of bromophenol blue solution R2. Not more than 0.05 mL of 0.1 M hydrochloric acid or 0.1 M sodium hydroxide is required to change the colour of the indicator.
Related substances
Examine by thin-layer chromatography (2.2.27), using silica gel G R as the coating substance.
Test solution Dissolve 0.5 g of the substance to be examined in methanol R and dilute to 10 mL with the same solvent.
Reference solution Dilute 0.1 mL of the test solution to 20 mL with methanol R.
Apply separately to the plate 2 µL of each solution. Develop over a path of 15 cm using a mixture of 40 volumes of ethyl acetate R and 60 volumes of hexane R. Allow the plate to dry in air for 15 min and expose it to iodine vapour. Any spot in the chromatogram obtained with the test solution, apart from the principal spot, is not more intense than the spot in the chromatogram obtained with the reference solution (0.5 per cent).
Pyrocatechol
To 2 mL of solution S add 1 mL of ammonium molybdate solution R2 and mix. Any yellow colour in the solution is not more intense than that in a standard prepared at the same time in the same manner using 2 mL of a 0.1 g/L solution of pyrocatechol R.
Loss on drying (2.2.32)
Not more than 1.0 per cent, determined on 1.00 g of powdered substance by drying in a desiccator for 4 h.
Sulfated ash (2.4.14)
Not more than 0.1 per cent, determined on 1.0 g.
ASSAY
Dissolve 0.500 g in water R and dilute to 250.0 mL with the same solvent. To 25.0 mL of the solution in a ground-glass- stoppered flask add 1.0 g of potassium bromide R, 50.0 mL of 0.0167 M potassium bromate, 15 mL of chloroform R and 15.0 mL of hydrochloric acid R1. Stopper the flask, shake and allow to stand in the dark for 15 min, shaking occasionally. Add 10 mL of a 100 g/L solution of potassium iodide R, shake thoroughly, allow to stand for 5 min and titrate with 0.1 M sodium thiosulfate, using 1 mL of starch solution R as indicator.
1 mL of 0.0167 M potassium bromate is equivalent to 1.835 mg of C6H6O2.
STORAGE
Store protected from light.