Edition: BP 2025 (Ph. Eur. 11.6 update)
Preparation
Menthol and Benzoin Inhalation
DEFINITION
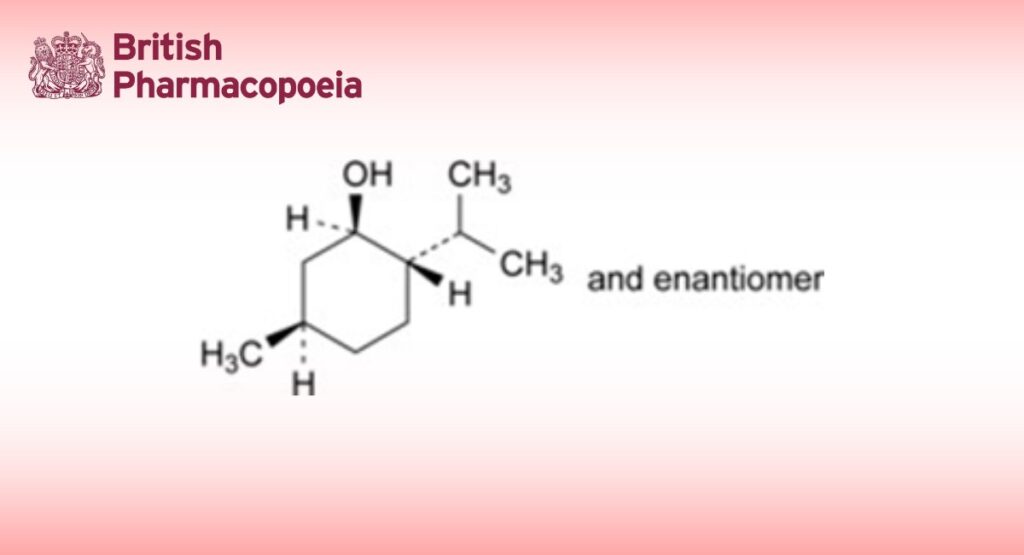
Mixture of equal parts of (1RS,2SR,5RS)-5-methyl-2-(1-methylethyl)cyclohexanol.
CHARACTERS
Appearance
Free-flowing or agglomerated, crystalline powder or prismatic or acicular, colourless, shiny crystals.
Solubility
Practically insoluble in water, very soluble in ethanol (96 per cent) and in light petroleum, freely soluble in fatty oils and in liquid paraffin, very slightly soluble in glycerol.
mp
About 34 °C.
IDENTIFICATION
First identification: A, C.
Second identification: B, D.
A. Optical rotation (see Tests).
B. Thin-layer chromatography (2.2.27).
Test solution Dissolve 25 mg of the substance to be examined in methanol R and dilute to 5 mL with the same solvent.
Reference solution Dissolve 25 mg of menthol CRS in methanol R and dilute to 5 mL with the same solvent.
Plate TLC silica gel G plate R.
Mobile phase ethyl acetate R, toluene R (5:95 V/V). Application 2 µL.
Development Over a path of 15 cm.
Drying In air, until the solvents have evaporated.
Detection Spray with anisaldehyde solution R and heat at 100-105 °C for 5-10 min.
Results The principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with the reference solution.
C. Examine the chromatograms obtained in the test for related substances.
Results The principal peak in the chromatogram obtained with test solution (b) is similar in position and approximate dimensions to the principal peak in the chromatogram obtained with reference solution (c).
D. Dissolve 0.20 g in 0.5 mL of anhydrous pyridine R. Add 3 mL of a 150 g/L solution of dinitrobenzoyl chloride R in anhydrous pyridine R. Heat on a water-bath for 10 min. Add 7.0 mL of water R in small quantities with stirring and allow to stand in iced water for 30 min. A precipitate is formed. Allow to stand and decant the supernatant. Wash the precipitate with 2 quantities, each of 5 mL, of iced water R, recrystallise from 10 mL of acetone R, wash with iced acetone R and dry at 75 °C at a pressure not exceeding 2.7 kPa for 30 min. The crystals melt (2.2.14) at 130 °C to 131 °C.
TESTS
Solution S
Dissolve 2.50 g in 10 mL of ethanol (96 per cent) R and dilute to 25.0 mL with the same solvent.
Appearance of solution
Solution S is clear (2.2.1) and colourless (2.2.2, Method II).
Acidity or alkalinity
Dissolve 1.0 g in ethanol (96 per cent) R and dilute to 10 mL with the same solvent. Add 0.1 mL of phenolphthalein solution R. The solution is colourless. Not more than 0.5 mL of 0.01 M sodium hydroxide is required to change the colour of the indicator to pink.
Optical rotation (2.2.7)
-0.2° to + 0.2°, determined on solution S.
Related substances
Gas chromatography (2.2.28).
Test solution (a) Dissolve 0.20 g of the substance to be examined in methylene chloride R and dilute to 50.0 mL with the same solvent.
Test solution (b) Dilute 1.0 mL of test solution (a) to 10.0 mL with methylene chloride R.
Reference solution (a) Dissolve 40.0 mg of the substance to be examined and 40.0 mg of isomenthol R in methylene chloride R and dilute to 100.0 mL with the same solvent.
Reference solution (b) Dilute 0.10 mL of test solution (a) to 100.0 mL with methylene chloride R.
Reference solution (c) Dissolve 40.0 mg of menthol CRS in methylene chloride R and dilute to 100.0 mL with the same solvent.
Column:
— material: glass;
— size: l = 2.0 m, Ø = 2 mm;
— stationary phase: diatomaceous earth for gas chromatography R impregnated with 15 per cent m/m of macrogol 1500 R.
Carrier gas nitrogen for chromatography R. Flow rate 30 mL/min.
Temperature:
— column: 120 °C;
— injection port: 150 °C;
— detector: 200 °C. Detection Flame ionisation. Injection 1 µL.
Run time Twice the retention time of menthol.
System suitability:
— resolution: minimum 1.4 between the peaks due to menthol and isomenthol in the chromatogram obtained with reference solution (a);
— signal-to-noise ratio: minimum 5 for the principal peak in the chromatogram obtained with reference solution (b).
Limits Test solution (a):
— total: not more than 1 per cent of the area of the principal peak;
— disregard limit: 0.05 per cent of the area of the principal peak.
Residue on evaporation
Maximum 0.05 per cent.
Evaporate 2.00 g on a water-bath and heat in an oven at 100-105 °C for 1 h. The residue weighs not more than 1.0 mg.